| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
EGFR; mTOR
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:大黄酸 (Chrysophanol) 是一种 EGFR/mTOR 通路抑制剂。大黄酸 (Chrysophanol) 是一种天然蒽醌,在 EGFR 过表达的 SNU-C5 人结肠癌细胞中具有抗癌活性。大黄酸 (Chrysophanol) 优先阻止 SNU-C5 细胞的增殖,但不会阻止其他 EGFR 表达水平较低的细胞系(HT7、HT29、KM12C、SW480、HCT116 和 SNU-C4)的增殖。 SNU-C5 细胞中的大黄酸 (Chrysophanol) 处理可抑制 EGF 诱导的 EGFR 磷酸化,并抑制下游信号分子的激活,例如 AKT、细胞外信号调节激酶 (ERK) 和哺乳动物雷帕霉素靶标 (mTOR)/核糖体蛋白S6 激酶 (p70S6K)。大黄酸还抑制 2 型和 3 型脊髓灰质炎病毒(小核糖核酸病毒科)的复制以及 BGM(水牛绿猴)肾细胞中脊髓灰质炎病毒诱导的细胞病变效应。细胞测定:将细胞以 5×103 个细胞/mL 接种在 96 孔微孔板中,并贴壁 24 小时。将大黄酚(20、50、80 和 120 μM)以不同的浓度(最高 120 μM)添加到培养基中并添加不同的持续时间。处理后,通过细胞计数试剂盒-8 (CCK-8) 评估细胞毒性和/或增殖。简而言之,高水溶性四唑盐 WST-8 产生橙色水溶性产物甲臜。细胞内脱氢酶产生的甲臜染料量与活细胞数量成正比。每孔加入CCK-8(10 μL),37℃孵育3 h,然后通过以下方法评估细胞增殖和细胞毒性:使用酶标仪测量 450 nm 处的吸光度。每个实验条件使用三个重复孔
|
||
| 体内研究 (In Vivo) |
大黄酚 (CA) 可改善 C57BL/6 小鼠 HFD 诱导的肥胖。在雄性 C57BL/6J 小鼠中进行大黄酚的体内性能以确定所施用的大黄酚的功效。喂食 HFD 的小鼠体重明显高于喂食标准饮食的小鼠。另一方面,大黄酚组的体重增加明显小于未处理的HFD。 16周后,HFD组的小鼠体重增加了23.92±1.74克,而大黄酚组的小鼠体重增加了16.72±2克
|
||
| 细胞实验 |
在 96 孔微孔板中,细胞以 5×103 细胞/mL 接种,并给予 24 小时贴壁。培养基中添加了不同浓度(最高 120 μM)的大黄酚(20、50、80 和 120 μM),添加时间也不同。 Cell Counting Kit-8 用于测量处理细胞 (CCK-8) 的细胞毒性和/或增殖。简而言之,甲臜是一种橙色水溶性产品,由高水溶性四唑盐 WST-8 生产。活细胞的数量与细胞脱氢酶产生的甲臜染料的量成正比。每孔加入10 μL CCK-8,37℃放置3小时后,用酶标仪测定450 nm处的吸光度,以确定细胞的细胞毒性和增殖情况。对于每个实验条件,使用三个重复孔[1]。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
A comparative oral pharmacokinetic study of five anthraquinones (aloe-emodin, emodin, rhein, chrysophanol and physcion) from the extract of Rheum palmatum L. was performed in normal and thrombotic focal cerebral ischemia (TFCI)-induced rats. The plasma samples were clarified through solid phase extraction prior to simultaneous determination of the anthraquinones with a validated high-performance liquid chromatography-fluorescence system. The results indicated that the Cmax, t(1/2) and AUC(0-t), of aloe-emodin, rhein, emodin and chrysophanol in TFCI-induced rats were nearly double, whereas the CL values were remarkably decreased (p < 0.05) over those of the normal rats. The plasma drug concentration-time data of five anthraquinones to rats fitted a two-compartment open model. The five anthraquinones in rat plasma were absorbed quickly and eliminated slowly in both groups. The obtained results could be helpful for evaluating the impact of the efficacy and safety of the drug in clinical applications. ETHNOPHARMACOLOGICAL RELEVANCE: Quyu Qingre granules (QYQRGs) are useful traditional Chinese composite prescription in the treatment of blood stasis syndrome. Comparing differences of pharmacokinetic properties of compounds in QYQRG between normal and blood stasis syndrome rabbits can provide much helpful information. The primary objective of this study was to compare the pharmacokinetics of rhein and chrysophanol after orally administering 2.0 g/kg b.w. QYQRG in normal and acute blood stasis model rabbits. MATERIALS AND METHODS: The blood samples were collected subsequently at 5, 10, 15, 20, 30, 45, 60, 75, 90, 120, 240, 360 and 480 min after orally administrating QYQRG. The concentrations of rhein and chrysophanol in rabbit plasma were determined by HPLC and main pharmacokinetic parameters were obtained. RESULTS: The pharmacokinetic parameters AUC(0-infinity), T(lag), Cmax and K21 of both rhein and chrysophanol were markedly different in the acute blood stasis model rabbits. It was also found that parameters A, beta, MRT and T(1/2beta) of rhein and the parameters a and T1/2a of chrysophanol all exhibited significant difference between the normal and acute blood stasis model rabbits. CONCLUSIONS: The absorption time of rhein and chrysophanol was accelerated and the absorption amount of these two compounds was increased in rabbits with acute blood stasis, suggesting that rhein and chrysophanol would possibly be the two effective compounds in QYQRG. AIM OF THE STUDY: The present study comparatively investigated the tissue distributions of rhubarb anthraquinone derivatives (AQs) to examine whether they undergo different uptakes in normal or CCl(4)-induced liver-damaged rats, to explore possible reasons for the different toxicities of AQs in pathological model rats and normal rats at the tissue distribution level. MATERIALS AND METHODS: The total rhubarb extract (14.49 g/kg of body weight per day based on the quantity of crude material) was administrated orally to normal and model rats for 12 weeks. The concentrations of free AQs in tissues were quantitated by liquid chromatography-tandem mass spectrometry (LC-MS). After drug withdrawal for 4 weeks, tissue distributions were again determined. RESULTS: The five free AQs-aloe-emodin, rhein, emodin, chrysophanol and physcion-were detected in the liver, kidney and spleen, while only rhein, aloe-emodin and emodin reached the quantitative limit. The tissue distributions of rhein (p < 0.001), aloe-emodin (p < 0.001) and emodin (p < 0.05) in normal rats were higher than those in model rats with rhein>aloe-emodin>emodin in kidney and spleen tissues and aloe-emodin > rhein > emodin in liver tissues. Free AQs were not detected in the tissues after drug withdrawal for 4 weeks. CONCLUSIONS: These results suggest that the tissue toxicity of AQs in normal animals is higher than that in pathological model animals with little accumulative toxicity of rhubarb. The results are concordant with the traditional Chinese theory of You Gu Wu Yun recorded first in Su Wen, a classical Chinese medical treatise. Metabolism / Metabolites The studies presented here were designed to elucidate the enzymes involved in the biotransformation of naturally occurring 1, 8-dihydroxyanthraquinones and to investigate whether biotransformation of 1,8-dihydroxyanthraquinones may represent a bioactivation pathway. We first studied the metabolism of emodin (1, 3,8-trihydroxy-6-methylanthraquinone), a compound present in pharmaceutical preparations. With rat liver microsomes, the formation of two emodin metabolites, omega-hydroxyemodin and 2-hydroxyemodin, was observed. The rates of formation of omega-hydroxyemodin were not different with microsomes from rats that had been pretreated with inducers for different cytochrome P450 enzymes. Thus, the formation of omega-hydroxyemodin seems to be catalyzed by several cytochrome P450 enzymes at low rates. The formation of 2-hydroxyemodin was increased in liver microsomes from 3-methylcholanthrene-pretreated rats and was inhibited by alpha-naphthoflavone, by an anti-rat cytochrome P450 1A1/2 antibody, and, to a lesser degree, by an anti-rat cytochrome P450 1A1 antibody. These data suggest the involvement of cytochrome P450 1A2 in the formation of this metabolite. However, other cytochrome P450 enzymes also seem to catalyze this reaction. The anthraquinone chrysophanol (1,8-dihydroxy-3-methylanthraquinone) is transformed, in a cytochrome P450-dependent oxidation, to aloe-emodin (1, 8-dihydroxy-3-hydroxymethylanthraquinone) as the major product formed. The mutagenicity of the parent dihydroxyanthraquinones and their metabolites was compared in the in vitro micronucleus test in mouse lymphoma L5178Y cells. 2-Hydroxyemodin induced much higher micronucleus frequencies, compared with emodin. omega-Hydroxyemodin induced lower micronucleus frequencies, compared with emodin. Aloe-emodin induced significantly higher micronucleus frequencies than did chrysophanol. These data indicate that the cytochrome P450-dependent biotransformation of emodin and chrysophanol may represent bioactivation pathways for these compounds. Chrysophanol, a major anthraquinone component occurring in many traditional Chinese herbs, is accepted as important active component with various pharmacological actions such as antibacterial and anticancer activity. Previous studies demonstrated that exposure to chrysophanol induced cytotoxicity, but the mechanisms of the toxic effects remain unknown. In the present metabolism study, three oxidative metabolites (M1-M3, aloe-emodine, 7-hydroxychrysophanol, and 2-hydroxychrysophanol) and five GSH conjugates (M4-M8) were detected in rat and human liver microsomal incubations of chrysophanol supplemented with GSH, and the formation of the metabolites was NADPH dependent except M4 and M5. M4 and M5 were directly derived from parent compound chrysophanol, M6 arose from M2, and M7 and M8 resulted from the oxidation of M4 and M5. Metabolites M5 and M6 were also observed in bile of rats after exposure to chrysophanol, M1-M3 and one NAC conjugate (M9) were detected in urine of rats administrated chrysophanol, and urinary metabolite M9 originated from the degradation of biliary GSH conjugation M6. Recombinant P450 enzyme incubation and microsome inhibition studies demonstrated that P450 1A2 was the primary enzyme responsible for the metabolic activation of chrysophanol and that P450 2B6 and P450 3A4 also participated in the generation of the oxidative metabolites. ... |
||
| 参考文献 | |||
| 其他信息 |
Chrysophanic acid appears as golden yellow plates or brown powder. Melting point 196 °C. Slightly soluble in water. Pale yellow aqueous solutions turn red on addition of alkali. Solutions in concentrated sulfuric acid are red. (NTP, 1992)
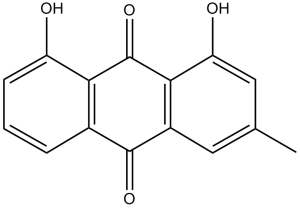
Chrysophanol is a trihydroxyanthraquinone that is chrysazin with a methyl substituent at C-3. It has been isolated from Aloe vera and exhibits antiviral and anti-inflammatory activity. It has a role as an antiviral agent, an anti-inflammatory agent and a plant metabolite. It is functionally related to a chrysazin. Chrysophanol has been reported in Talaromyces islandicus, Ramularia uredinicola, and other organisms with data available. See also: Frangula purshiana Bark (part of). |
| 分子式 |
C15H10O4
|
|
|---|---|---|
| 分子量 |
254.24
|
|
| 精确质量 |
254.057
|
|
| 元素分析 |
C, 70.86; H, 3.96; O, 25.17
|
|
| CAS号 |
481-74-3
|
|
| 相关CAS号 |
|
|
| PubChem CID |
10208
|
|
| 外观&性状 |
Yellow to orange solid powder
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 沸点 |
489.5±45.0 °C at 760 mmHg
|
|
| 熔点 |
194-198 °C
|
|
| 闪点 |
263.9±25.2 °C
|
|
| 蒸汽压 |
0.0±1.3 mmHg at 25°C
|
|
| 折射率 |
1.710
|
|
| LogP |
5.03
|
|
| tPSA |
74.6
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
0
|
|
| 重原子数目 |
19
|
|
| 分子复杂度/Complexity |
405
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O([H])C1=C([H])C(C([H])([H])[H])=C([H])C2C(C3C([H])=C([H])C([H])=C(C=3C(C=21)=O)O[H])=O
|
|
| InChi Key |
LQGUBLBATBMXHT-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C15H10O4/c1-7-5-9-13(11(17)6-7)15(19)12-8(14(9)18)3-2-4-10(12)16/h2-6,16-17H,1H3
|
|
| 化学名 |
1,8-dihydroxy-3-methylanthracene-9,10-dione
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.9333 mL | 19.6665 mL | 39.3329 mL | |
| 5 mM | 0.7867 mL | 3.9333 mL | 7.8666 mL | |
| 10 mM | 0.3933 mL | 1.9666 mL | 3.9333 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。