| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
Luminescent enzyme substrate
|
|---|---|
| 体外研究 (In Vitro) |
基于生物发光的技术,如生物发光成像,BRET和双荧光素酶报告分析系统,已被广泛用于检查无数的生物过程。Coelenterazine (CTZ)是一种在生物发光生物中发现的荧光素或发光化合物,引起了人们对寻找具有改进光化学性质的类似物的极大好奇心和兴趣。本文综述了目前coelenterazine类似物的研究进展、其生物发光特性、笼型coelenterazine对生物靶点的合理设计及其在生物检测中的应用。需要强调的是,笼型荧光素的设计可以为深入了解生物体的详细分子过程提供有价值的见解,将是生物发光分子发展的一个趋势。[1]
|
| 酶活实验 |
笼状coelenterazine衍生物作为研究酶活性的生物发光探针。生物发光成像(BLI)正在成为一项重要的、易于应用于生命科学的技术。腔肠-荧光素酶系统,除了分子氧外,不需要额外的辅助因子,比萤火虫荧光素酶系统或细菌系统更简单。因此,coelenterazine衍生物可以作为生物发光探针来监测和成像体外和体内的事件。2013年,菊池和也(Kazuya Kikuchi)实验室报告了两种笼型铜肠嗪衍生物132和133(图11)。它们被设计和合成用于在表达突变型高斯荧光素酶的HEK-293T细胞培养中成像β-半乳糖苷酶的活性和表达。这些化合物在生物发光的关键位点(咪唑吡嗪酮部分的羰基)上引入β-半乳糖苷酶可切割的笼状基团,从而降低了coelenterazine的自氧化性,并且对β-半乳糖苷酶具有很高的特异性。笼型胶肠嘧啶探针本身不发出荧光素酶的生物发光。然而,在β-半乳糖苷酶存在下,含有β-半乳糖苷酶可切割基团的探针被酶切割,产生游离的胶肠嗪与高斯荧光素酶反应,产生生物发光信号(图11)。此外,化合物133在表达β- gal的细胞中可以产生游离的coelenterazine。此外,只有当它容易扩散到表达glucm23的细胞中,并与外膜结合葡萄糖或细胞内定位葡萄糖时,它才会产生生物发光(图11)。换句话说,探针只有在被β-半乳糖苷酶切割并遇到荧光素酶时才会产生生物发光。因此,化合物133具有在两种不同细胞群中作为双重报告基因的潜力这是第一个基于笼型铜肠嘧啶策略成功评估酶活性的探针。该研究为进一步研究基于钴肠嘧啶的生物发光探针及其应用提供了参考。在咪唑吡嗪酮核心的C-3位置很难引入较大的笼化基团。因此,这是一个很好的例子,可以指导笼状腔肠嘧啶型探针的设计。[1]
|
| 细胞实验 |
2013年,Kikuchi实验室报道了第一个不透膜的coelenterazine衍生物(图13),化合物145,它有可能被用作监测细胞膜融合事件的生物发光探针(图14)85该化合物是用含有末端阴离子膦酸酯部分的连接剂将钴肠嗪烷基化而合成的。同时,合成了另外两个含有末端苯基保护基团的聚乙二醇(PEG)连接剂的coelenterazine衍生物143和144,以探讨其对生物发光的影响。但是,它们的性能不如探针145。探针145由于其负电荷而具有高度的细胞不渗透性,导致无法穿透细胞膜与细胞内定位的葡萄糖发生反应。而探针145可以通过外膜结合的GlucM23Mem发出生物发光信号。此外,如果携带Gluc的分泌囊泡与细胞膜融合,则Gluc将暴露于探针145,从而产生生物发光。此外,有趣的是,化合物145对Gaussia luciferase (Gluc)的生物发光活性比Renilla luciferase (RLuc)高30倍,这表明Gluc更合适。考虑到coelenterazine型探针的较低应用,该探针是使用小分子探针探索生物过程的一个例子。[1]
|
| 参考文献 | |
| 其他信息 |
The development of coelenterazine is focused on two main directions. One strategy is traditional modification of the coelenterazine substrate, especially the optimization of substitution at the C-2, C-6 and C-8 positions of the imidazopyrazinone core. Much work has been done in the past two decades in order to discover more suitable compounds with increased luminescence output, red-shifted emission and high stability of bioluminescence. Overall, the introduction of electron donating groups into the C-2, C-6 and C-8 positions which made the molecule more polar or increased the conjugation degree made a significant contribution to improving the bioluminescence intensity or influenced the maximum peak of emission. However, only a few of these compounds displayed excellence in all aspects that could be applied to bioluminescence assays, taking the place of native coelenterazine. The other strategy is to cage the 3-carbonyl position of the imidazopyrazinone skeleton of coelenterazine, which forms the critical active site of the bioluminescence reaction. The caged coelenterazine derivatives with high stability can emit bioluminescence only in particular situations. Therefore, the caged coelenterazine derivatives could be used as slow release substrates or as bioluminescent probes to detect biomacromolecules and bioactive small molecules. Very recently, some caged firefly luciferins as bioluminescent probes have been reported, and some of them display remarkable behavior.4,88–95 However, only a handful of studies have been done on caged coelenterazine. The reason why they have seen so little application may be that the yields of the caged reactions reported were very low. The explanation may be that it is difficult to cage the 3-carbonyl of the imidazopyrazinone ring due to its sensitivity to base and oxygen. Moreover, coelenterazine and its derivatives seem to be more unstable compared with firefly luciferins, which could be the main drawback of its application. Another disadvantage of coelenterazine is that its light emission maximum is less than 600 nm, which makes it inappropriate for deep tissue imaging. However, the coelenterazine bioluminescence system still has obvious advantages. This universal luciferin, coelenterazine, could be used in numerous bioluminescent luciferase systems, such as Renilla and Gaussia. Moreover, most of the coelenterazines involved a bioluminescence system that only consists of luciferin and luciferase without other constituents, which is much simpler. So far, the application of luciferin–luciferase bioluminescence has been broadly extended to a variety of fields in the chemobiological sciences. It should be emphasized that the design of caged luciferins can provide valuable insight into the detailed molecular processes of organisms and represents a new trend in the development of bioluminescent molecules. Caged coelenterazine probes, an important class of bioluminescent molecules, have the potential to become popular in future research fields. Meanwhile, novel strategies and techniques should be proposed and developed to promote the application of coelenterazine-type bioluminescence.[1]
|
| 分子式 |
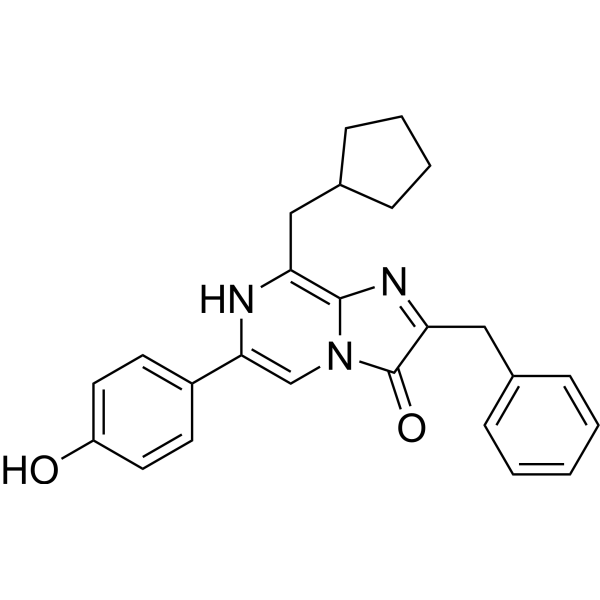
C25H25N3O2
|
|---|---|
| 分子量 |
399.4849
|
| 精确质量 |
399.195
|
| CAS号 |
123437-32-1
|
| PubChem CID |
135439140
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.3 g/cm3
|
| 沸点 |
568.2ºC at 760 mmHg
|
| 闪点 |
297.4ºC
|
| 蒸汽压 |
1.63E-13mmHg at 25°C
|
| 折射率 |
1.685
|
| LogP |
4.718
|
| tPSA |
70.39
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
541
|
| 定义原子立体中心数目 |
0
|
| SMILES |
C1=CC=C(C=C1)CC2=NC3=C(CC4CCCC4)NC(=CN3C2=O)C5=CC=C(C=C5)O
|
| InChi Key |
UCSBOFLEOACXIR-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C25H25N3O2/c29-20-12-10-19(11-13-20)23-16-28-24(21(26-23)14-17-8-4-5-9-17)27-22(25(28)30)15-18-6-2-1-3-7-18/h1-3,6-7,10-13,16-17,29-30H,4-5,8-9,14-15H2
|
| 化学名 |
2-benzyl-8-(cyclopentylmethyl)-6-(4-hydroxyphenyl)imidazo[1,2-a]pyrazin-3-ol
|
| 别名 |
2-benzyl-8-(cyclopentylmethyl)-6-(4-hydroxyphenyl)-7H-imidazo[1,2-a]pyrazin-3-one; CLZN-hcp; 2-benzyl-8-(cyclopentylmethyl)-6-(4-hydroxyphenyl)imidazo[1,2-a]pyrazin-3-ol; Coelenterazine hcp, solid; SCHEMBL14117493; DTXSID40376337;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5033 mL | 12.5163 mL | 25.0325 mL | |
| 5 mM | 0.5007 mL | 2.5033 mL | 5.0065 mL | |
| 10 mM | 0.2503 mL | 1.2516 mL | 2.5033 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。