| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The oral bioavailability of cyclobenzaprine has been estimated to be between 0.33 and 0.55. Cmax is between 5-35 ng/mL and is achieved after 4 hours (Tmax). AUC over an 8 hour dosing interval was reported to be approximately 177 ng.hr/mL. After administration of a radio-labeled dose of cyclobenzaprine, 38-51% of radioactivity was excreted in the urine while 14-15% was excreted in the feces. Cyclobenzaprine is highly metabolized, with only approximately 1% of this same radio-labeled dose recovered in the urine as unchanged drug. Metabolites excreted in the urine are likely water-soluble glucuronide conjugates. The volume of distribution of cyclobenzaprine is approximately 146 L. The combination of high plasma clearance despite a relatively long half-life observed with cyclobenzaprine is suggestive of extensive tissue distribution. The approximate plasma clearance of cyclobenzaprine is 0.7 L/min. Cyclobenzaprine is widely distributed into body tissues. ... It is not known if cyclobenzaprine crosses the placenta. The drug is extensively (about 93%) bound to plasma protein. /MILK/ It is not known if cyclobenzaprine is distributed into milk in humans; however, the drug is distributed into milk in rats. The absorption, distribution, excretion, and metabolism of 3-(5 H-dibenzo[a,d]cyclohepten-5-ylidene)-N,N-dimethyl-1-propanamine (cyclobenzaprine) were investigated in the rat, dog, rhesus monkey, and man. The drug was well absorbed in all species after oral administration. The rat eliminated the drug primarily in the feces, but urinary excretion was predominant in the dog, monkey, and man. The drug was rapidly and widely distributed into rat tissues, highest concentrations being found in the small intestine, lung, kidney, and liver. The drug was highly bound in human plasma. Extensive biliary excretion of the labeled compound was observed in the rat. Major metabolites in the rat were phenolic derivatives but in man the major metabolites were 10,11-dihydroxynortriptyline and cyclobenzaprine glucuronide. Only minor amounts of unchanged drug were present in the urine. Orally administered cyclobenzaprine is well absorbed. Cyclobenzaprine undergoes enterohepatic circulation, and appears to be metabolized during its first pass through the GI tract and/or liver. Mean oral bioavailability of the drug is estimated to range from 33-55%. Following oral administration of a single 5- or 10-mg dose of cyclobenzaprine hydrochloride, peak plasma concentrations of 4.3 or 8.5 ng/mL, respectively, are attained in about 4 hours. When cyclobenzaprine is administered 3 times daily, steady-state plasma concentrations are attained within 3-4 days that are about fourfold greater than those after a single dose. In healthy individuals receiving the drug 3 times daily, a mean steady-state peak plasma cyclobenzaprine concentration of 14.9 or 25.9 ng/mL was achieved at 4 or 3.9 hours after administration of a 5 or 10 mg dose, respectively. Metabolism / Metabolites Cyclobenzaprine is extensively metabolized in the liver via both oxidative and conjugative pathways. Oxidative metabolism, mainly N-demethylation, is catalyzed primarily by CYP3A4 and CYP1A2 (with CYP2D6 implicated to a lesser extent) and is responsible for the major metabolite desmethylcyclobenzaprine. Cyclobenzaprine also undergoes N-glucuronidation in the liver catalyzed by UGT1A4 and UGT2B10, and has been shown to undergo enterohepatic circulation. Ten metabolites of cyclobenzaprine, accounting for approximately 50% of the urinary radioactivity, were identified in the urine of dogs to which the labeled drug had been given orally. These included the 1,2-dihydrodiol, three phenolic derivatives, the N-oxide, the 10,11-epoxide, the 10,11-glycol, desmethylcyclobenzaprine, and the glucuronide conjugates of desmethylcyclobenzaprine and cyclobenzaprine. The metabolites were excreted in both the free and conjugated states. Unchanged cyclobenzaprine was present in only minor amounts. Cyclobenzaprine is extensively metabolized, and is excreted primarily as glucuronides via the kidney. Cytochromes P-450 3A4, 1A2, and, to a lesser extent, 2D6, mediate N-demethylation, one of the oxidative pathways for cyclobenzaprine. The absorption, distribution, excretion, and metabolism of 3-(5 H-dibenzo[a,d]cyclohepten-5-ylidene)-N,N-dimethyl-1-propanamine (cyclobenzaprine) were investigated in the rat, dog, rhesus monkey, and man. ... Major metabolites in the rat were phenolic derivatives but in man the major metabolites were 10,11-dihydroxynortriptyline and cyclobenzaprine glucuronide. ... Cyclobenzaprine is extensively metabolized by both oxidative and conjugative pathways. Hepatic cytochrome P-450 (CYP) 3A4, 1A2, and (to a lesser extent) 2D6 isoenzymes are responsible for oxidative N-demethylation of the drug.Orally administered cyclobenzaprine is excreted in urine principally as inactive glucuronide metabolites; less than 1% of the drug is excreted renally as unchanged drug. The fungus, Cunninghamella elegans, was used as a microbial model of mammalian drug metabolism to biotransform a tricyclic antidepressant, cyclobenzaprine. Seventy-five percent of this drug at a concentration of 1 mM was metabolized within 72 hr by C. elegans grown on Sabouraud dextrose broth. Milligram amounts of fungal metabolites were isolated by reversed-phase high performance liquid chromatography (HPLC) and their structures were characterized by 1H NMR spectroscopy, mass spectrometry, and UV spectroscopy analyses. The major fungal metabolites of cyclobenzaprine were 2-hydroxycyclobenzaprine (59%), N-desmethylcyclobenzaprine (21%), cyclobenzaprine trans-10,11-dihydrodiol (5%), N-desmethyl-2-hydroxy-cyclobenzaprine (3%), 3-hydroxycyclobenzaprine (3%), and cyclobenzaprine N-oxide (1%). These fungal metabolites were used as standards to investigate the metabolism of cyclobenzaprine by rat liver microsomes. Rat liver microsomes also biotransformed cyclobenzaprine to produce similar metabolites as the fungus. The isotope labeling of 2-hydroxycyclobenzaprine by 18O2 and the trans-configuration of the dihydrodiol suggested that these reactions were catalyzed by cytochrome P-450 monooxygenases in C. elegans. These results also demonstrated that the fungal biotransformation system could be used to predict and synthesize the mammalian drug metabolites. Cyclobenzaprine has known human metabolites that include N-Desmethylcyclobenzaprine. Biological Half-Life The effective half-life of cyclobenzaprine in young healthy subjects is approximately 18 hours. These values are extended in the elderly and those with hepatic insufficiency, with a mean effective half-life of 33.4 hours and 46.2 hours in these groups, respectively. Cyclobenzaprine is eliminated quite slowly, with an effective half-life of 18 hours (range 8-37 hours; n=18) ... . |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Cyclobenzaprine (used in the form of cyclobenzaprine hydrochloride tablets) is indicated as an adjunct to rest and physical therapy for relief of muscle spasm associated with acute, painful musculoskeletal conditions. HUMAN EXPOSURE AND TOXICITY: Manifestations of toxicity may develop rapidly after a cyclobenzaprine overdose, and rarely, death may occur. The most common toxic effects associated with cyclobenzaprine overdose are drowsiness and tachycardia; less frequent manifestations include tremor, agitation, coma, ataxia, hypertension, slurred speech, confusion, dizziness, nausea, vomiting, and hallucinations. Rarely, potentially serious effects may include cardiac arrest, chest pain, cardiac dysrhythmias, severe hypotension, seizures, and neuroleptic malignant syndrome. Serotonin syndrome is another potential side effect. Blood concentration of > or = 0.8 mg/L cyclobenzaprine may be associated with a fatal outcome. In one case of accidental overdose, the victim developed severe hypothermia and then developed cardiac arrest during transport. ANIMAL STUDIES: Ptyalism, emesis, tremors, convulsions and increased respiratory rate developed and death occurred within 1 hour following single oral doses of 180 mg/kg or more by gavage in dogs. Acute exposure to the drug in rats resulted in ataxia, decreased respiratory rate, sedation, flaccid hind legs, loss of the ear flick reflex, loss of the righting reflex with swimming movements, intermittent clonic convulsions, weight loss, lethargy, and then death. The drug was more toxic to infant and weanling rats than to young adults. In rats treated with cyclobenzaprine hydrochloride for up to 67 weeks at doses of approximately 5 to 40 times the maximum recommended human dose, pale, sometimes enlarged, livers were noted and there was a dose-related hepatocyte vacuolation with lipidosis. No evidence of embryo lethality or teratogenicity was revealed following oral doses of 5, 10 or 20 mg/kg/day in studies in mice and rabbits. The reproductive performance and fertility of males and females, and the growth and survival of their offspring were not adversely affected by doses of 5 or 10 mg/kg/day in rats. Litter size, size and survival of the pups, and weight gain of the mothers were decreased by doses of 20 mg/kg/day. Cyclobenzaprine hydrochloride was determined to have no genotoxic effects in several assays, including mouse bone marrow micronucleus assay; Salmonella-Escherichia coli mammalian microsome reverse mutation assay with confirmatory assay; and in a CHO cells chromosomal aberration assay with and without metabolic activation. Hepatotoxicity The product insert for cyclobenzaprine mentions that abnormal liver function, hepatitis, jaundice, and cholestasis occur in Likelihood score: E* (Unproven but suspected cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Amounts of cyclobenzaprine in milk appear to be very small and two infants apparently tolerated the drug in milk well. If cyclobenzaprine is required by the mother, it is not a reason to discontinue breastfeeding. Monitor the infant for (drowsiness, adequate weight gain, and developmental milestones), especially in neonates and preterm infants and when using combinations of sedating drugs. ◉ Effects in Breastfed Infants Two mothers were taking chronic cyclobenzaprine therapy. One mother was taking 5 mg once daily for temporomandibular joint pain and the other mother was taking 10 mg twice daily for fibromyalgia pain. The latter mother was also taking unspecified antidepressants, levothyroxine, zolpidem, alprazolam and famotidine. Both mothers were breastfeeding their infants (extent not stated). Neither infant had any noticeable adverse reaction such as sedation. A search was performed of the shared database of all U.S. poison control centers for the time period of 2001 to 2017 for calls regarding medications and breastfeeding. Of 2319 calls in which an infant was exposed to a substance via breastmilk, 1 of 7 classified as resulting in a major adverse effect involved cyclobenzaprine. A 16-day-old infant was exposed to cyclobenzaprine, acetaminophen and oxycodone in breastmilk. The infant was admitted to the hospital in a noncritical care unit for bradycardia, hypotension, and respiratory arrest. The dosages and extent of breastfeeding were not reported and the infant survived. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Cyclobenzaprine is approximately 93% protein bound in plasma. It has been identified as specifically having a high affinity for human serum albumin. Interactions Concomitant use of cyclobenzaprine with diflunisal or naproxen reportedly was well tolerated and did not appear to result in any unexpected adverse effects. However, concomitant use of cyclobenzaprine with naproxen has been associated with an increased incidence of drowsiness. Plasma concentrations of aspirin or cyclobenzaprine were unaffected when the drugs were administered concomitantly. It has not been established whether combined therapy with cyclobenzaprine and aspirin (or other analgesics) will result in enhanced clinical efficacy. Cyclobenzaprine and structurally similar tricyclic antidepressants may block the hypotensive effects of guanethidine (no longer commercially available in the US) and other similarly acting drugs. Cyclobenzaprine and structurally similar tricyclic antidepressants may enhance the risk of seizures in patients receiving tramadol. Cyclobenzaprine may be additive with or may potentiate the action of other CNS depressants (e.g., alcohol, barbiturates). Cyclobenzaprine, especially when used concomitantly with alcohol or other CNS depressants, may impair the patient's ability to perform activities requiring mental alertness or physical coordination (e.g., operating machinery, driving a motor vehicle). For more Interactions (Complete) data for Cyclobenzaprine (9 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse iv 36 mg/kg LD50 Mouse ip 90 mg/kg LD50 Mouse oral 250 mg/kg LD50 Rat oral 425 mg/kg LD50 Mouse oral 338 mg/kg |
| 参考文献 |
Eur J Pharmacol.1996 Sep 5;311(1):29-35;Eur J Pharmacol.2003 Jan 1;458(1-2):91-9. |
| 其他信息 |
Therapeutic Uses
Antidepressive Agents, Tricyclic; Muscle Relaxants, Central; Tranquilizing Agents /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Cyclobenzaprine is included in the database. Cyclobenzaprine hydrochloride tablets are indicated as an adjunct to rest and physical therapy for relief of muscle spasm associated with acute, painful musculoskeletal conditions. Improvement is manifested by relief of muscle spasm and its associated signs and symptoms, namely, pain, tenderness, limitation of motion, and restriction in activities of daily living. Cyclobenzaprine hydrochloride should be used only for short periods (up to two or three weeks) because adequate evidence of effectiveness for more prolonged use is not available and because muscle spasm associated with acute, painful musculoskeletal conditions is generally of short duration and specific therapy for longer periods is seldom warranted. Cyclobenzaprine hydrochloride has not been found effective in the treatment of spasticity associated with cerebral or spinal cord disease, or in children with cerebral palsy. /Included in US product label/ Some data suggest that cyclobenzaprine may be useful for the treatment of fibrositis. Cyclobenzaprine is ineffective in the treatment of spasticity associated with cerebral or spinal disease or in children with cerebral palsy. /NOT included in US product label/ EXPL Tinnitus is defined as an intrinsic sound sensation that cannot be attributed to an external sound source. Currently there are no standardized drug therapies for the treatment of tinnitus. Based on the analogy between pain and tinnitus it is suggested that among all antidepressant families that have been used for tinnitus, particular interest should be paid to the tricyclic group of drugs as they have an analgesic effect. The aim of the present study was to investigate the effect of a tricyclic pharmacological agent, namely cyclobenzaprine for the relief of tinnitus complaints. 65 patients, who received the drug treatment, were compared to 30 patients on a waiting list, who received no treatment. Analysis shows that cyclobenzaprine offers some benefit to patients with tinnitus on both tinnitus intensity and tinnitus distress, while a waiting list control group does not demonstrate any improvement: 24% of the tinnitus patients showed a clear response to cyclobenzaprine with a reduction of 53% on tinnitus intensity and 25% had a clear response to cyclobenzaprine with a reduction of 55% on tinnitus distress. It was further demonstrated that particular subgroups, namely pure tone tinnitus patients and unilateral tinnitus patients, respond better to cyclobenzaprine. Our results indicate that cyclobenzaprine is a promising drug to treat tinnitus particularly in certain subgroups. As there is a good risk-benefit ratio and there are currently no well-established, specific treatments for tinnitus, cyclobenzaprine might be worthwhile to further investigate. Drug Warnings The development of a potentially life-threatening serotonin syndrome has been reported with Cyclobenzaprine Hydrochloride when used in combination with other drugs, such as selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), tramadol, bupropion, meperidine, verapamil, or MAO inhibitors. The concomitant use of Cyclobenzaprine Hydrochloride with MAO inhibitors is contraindicated. Serotonin syndrome symptoms may include mental status changes (e.g., confusion, agitation, hallucinations), autonomic instability (e.g., diaphoresis, tachycardia, labile blood pressure, hyperthermia), neuromuscular abnormalities (e.g., tremor, ataxia, hyperreflexia, clonus, muscle rigidity), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Treatment with Cyclobenzaprine Hydrochloride and any concomitant serotonergic agents should be discontinued immediately if the above reactions occur and supportive symptomatic treatment should be initiated. If concomitant treatment with Cyclobenzaprine Hydrochloride and other serotonergic drugs is clinically warranted, careful observation is advised, particularly during treatment initiation or dose increases. Dry mouth occurred in 21 or 32% of patients receiving 5 or 10 mg, respectively, of cyclobenzaprine and in 7% of those receiving placebo in controlled studies. Dry mouth also occurred in 27 or 7% of patients receiving 10 mg of the drug in clinical studies or during postmarketing surveillance, respectively. Abdominal pain, acid regurgitation, dyspepsia, constipation, diarrhea, nausea, and unpleasant taste occurred in 1-3% of patients receiving 5 or 10 mg of cyclobenzaprine in controlled studies or during postmarketing surveillance in patients receiving 10 mg of the drug. Vomiting, anorexia, GI pain, gastritis, thirst, edema of the tongue, and flatulence were reported during postmarketing surveillance or in less than 1% of patients receiving 10 mg of the drug in controlled studies. Paralytic ileus, tongue discoloration, stomatitis, and parotid swelling were reported in patients receiving other tricyclic drugs or rarely with cyclobenzaprine, but a causal relationship with cyclobenzaprine could not be established. Malaise, seizures, ataxia, vertigo, dysarthria, hypertonia, tremors, disorientation, insomnia, depressed mood, abnormal sensations, anxiety, agitation, psychosis, abnormal thinking, abnormal dreaming, hallucinations, excitement, paresthesia, and diplopia were reported during postmarketing surveillance or in less than 1% of patients receiving 10 mg of the drug in controlled studies. Other adverse nervous system effects that have been reported in patients receiving other tricyclic drugs or rarely with cyclobenzaprine but for which a causal relationship with the drug could not be established include decreased or increased libido, abnormal gait, delusions, aggressive behavior, paranoia, peripheral neuropathy, Bell's palsy, alterations in EEG patterns, and extrapyramidal manifestations. Headache occurred in 5% of those receiving 5 or 10 mg of cyclobenzaprine and in 8% of those receiving placebo in controlled studies; headache occurred in 1-3% of patients receiving 10 mg of the drug in controlled studies and postmarketing surveillance. Irritability, decreased mental acuity, nervousness, asthenia, and confusion occurred in 1-3% of patients receiving 5 or 10 mg of cyclobenzaprine in controlled studies or during postmarketing surveillance in patients receiving 10 mg of the drug. For more Drug Warnings (Complete) data for Cyclobenzaprine (25 total), please visit the HSDB record page. Pharmacodynamics Cyclobenzaprine is a skeletal muscle relaxant that works on areas of the brainstem to reduce skeletal muscle spasm, though its exact pharmacodynamic behaviour is currently unclear. Despite its long half-life, it is relatively short-acting with a typical duration of action of 4-6 hours. Cyclobenzaprine has been reported to contribute to the development of serotonin syndrome when used in combination with other serotonergic medications. Symptoms of serotonin syndrome may include autonomic instability, changes to mental status, neuromuscular abnormalities, or gastrointestinal symptoms - treatment with cyclobenzaprine should be discontinued immediately if any of these reactions occur during therapy. |
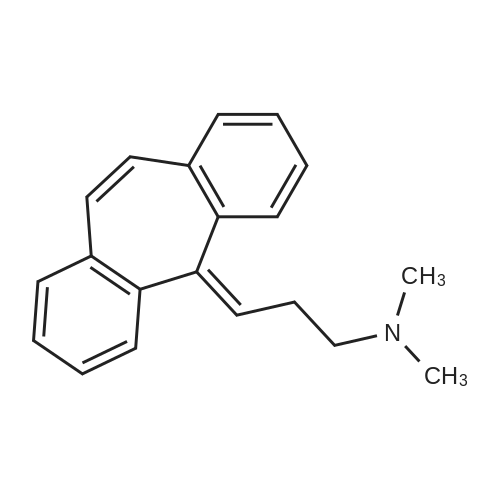
| 分子式 |
C20H21N
|
|---|---|
| 分子量 |
275.39
|
| 精确质量 |
275.167
|
| CAS号 |
303-53-7
|
| 相关CAS号 |
303-53-7;6202-23-9 (HCl);
|
| PubChem CID |
2895
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.096 g/cm3
|
| 沸点 |
405.9ºC at 760 mmHg
|
| 闪点 |
177.8ºC
|
| 折射率 |
1.7500 (estimate)
|
| LogP |
4.553
|
| tPSA |
3.24
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
21
|
| 分子复杂度/Complexity |
365
|
| 定义原子立体中心数目 |
0
|
| SMILES |
Cl.CN(CC/C=C1\C=C2C=CC=CC2=CC2=CC=CC=C\12)C
|
| InChi Key |
JURKNVYFZMSNLP-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C20H21N/c1-21(2)15-7-12-20-18-10-5-3-8-16(18)13-14-17-9-4-6-11-19(17)20/h3-6,8-14H,7,15H2,1-2H3
|
| 化学名 |
N,N-dimethyl-3-(2-tricyclo[9.4.0.03,8]pentadeca-1(15),3,5,7,9,11,13-heptaenylidene)propan-1-amine
|
| 别名 |
MK-130MK130Flexeril
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.6312 mL | 18.1561 mL | 36.3121 mL | |
| 5 mM | 0.7262 mL | 3.6312 mL | 7.2624 mL | |
| 10 mM | 0.3631 mL | 1.8156 mL | 3.6312 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。