| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
在 paVIC 中,丹曲林(60 μM;第 1 天和第 3 天)显着降低 ACTA2 表达并增加 RUNX2 表达 [2]。使用丹曲林(60 μM;睡眠)可抑制猪主动脉瓣间质细胞诱导 LPC。 10 μM 溶血磷脂酰胆碱 (LPC) 可导致 paVIC 钙化节点的形成,而丹曲林 (10、30、60 μM) 可以防止这种情况发生 [2]。
|
|---|---|
| 体内研究 (In Vivo) |
丹曲林(5 mg/kg;每周 3 次)可增强步态步行和平衡木步行测定性能 [3]。在 40-60 天的时间里,丹曲林(10 mg/kg;腹腔注射;每周三天)显着增强步态,降低 LC3-II 水平,增强线粒体 ATP 合成,并降低大脑调节。 Dantrolene 可降低患有神经性戈谢病的小鼠大脑中钙调蛋白 (CALM) 的表达和自噬 [4]。
|
| 细胞实验 |
RT-PCR[2]
细胞类型:猪钙化结节的形成[2]。主动脉瓣间质细胞 (paVIC) 测试浓度: 60 μM 孵育时间: 第 1 天和第 3 天 实验结果: 显着抑制 ACTA2 表达并上调 RUNX2 表达。 |
| 动物实验 |
Animal/Disease Models: YAC128 transgenic mice (FVBN/NJ background strain) and WT mice [3]
Doses: 5 mg/kg Route of Administration: Oral twice a week from 2 to 11.5 months of age Experimental Results: Significant improvement in balance beam walking and gait performance walking assay. Dramatically diminished the loss of NeuN-positive striatal neurons and diminished the formation of Httexp nuclear aggregates. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Bioavailability is 70%. ABSORPTION...FROM GI TRACT IS SLOW & INCOMPLETE BUT SUFFICIENTLY CONSISTENT TO PROVIDE DOSE-RELATED PLASMA CONCN. MEAN HALF LIFE OF DRUG IN ADULTS IS ABOUT 9 HR AFTER 100-MG DOSE. IT IS SLOWLY METABOLIZED BY LIVER, & THE 5-HYDROXY & ACETAMIDO METABOLITES ARE EXCRETED WITH UNCHANGED DRUG IN URINE. Metabolism / Metabolites Hepatic, most likely by hepatic microsomal enzymes. Its major metabolites in body fluids are 5-hydroxydantrolene and an acetylamino metabolite of dantrolene. Another metabolite with an unknown structure appears related to the latter. Dantrium may also undergo hydrolysis and subsequent oxidation forming nitrophenylfuroic acid. DANTROLENE IS METABOLIZED BY THE HEPATIC MIXED FUNCTION OXIDASE SYSTEM TO 5-HYDROXYDANTROLENE WHICH IS CONJUGATED WITH GLUCURONIC ACID OR WITH SULFATE. IT IS ALSO METABOLIZED BY NITROREDUCTASE TO AMINODANTROLENE WHICH INHIBITS THE HEPATIC MIXED FUNCTION OXIDASE SYSTEM. ACETYLATION OF AMINODANTROLENE BLOCKS THE INHIBITORY EFFECTS. INTERMEDIATES IN THE NITROREDUCTASE PATHWAY FORM GLUCURONIDE & MERCAPTURIC ACID CONJUGATES. THE MERCAPTURIC ACID CONJUGATION REACTION IS A DETOXIFICATION MECHANISM FOR AN ELECTROPHILIC METABOLITE OF DANTROLENE. Biological Half-Life The mean biologic half-life after intravenous administration is variable, between 4 to 8 hours under most experimental conditions, while oral is 8.7 hours for a 100mg dose. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Mild, asymptomatic serum aminotransferase elevations during dantrolene therapy are relatively uncommon (1%), but clinically over liver injury is estimated to occur in 1 to 2 per thousand treated persons (0.1% to 0.2%). The liver injury can be severe; cases of acute liver failure and even death have been described (Case 1). The latency to onset of clinically apparent liver injury ranges from one week to several months, but is usually within the first 6 months of starting therapy (Case 2). More serious cases are associated with a sudden onset with jaundice, nausea and fatigue, and rapid progression. Allergic manifestations such as fever, rash and eosinophilia are rare, as are autoimmune features. The pattern of enzyme elevations is predominantly hepatocellular. Liver histology demonstrates an acute-hepatitis like picture. Recovery is usually complete within 1 to 3 months. Women, the elderly, and patients taking higher doses appear to be more susceptible to developing dantrolene hepatotoxicity. Likelihood score: A (Well established cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because no information is available on the long-term use of dantrolene during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. After short-term use, the drug would be expected to be eliminated from milk in 1 to 2 days. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Significant, mostly to albumin. Interactions CENTRAL EFFECTS OF DANTROLENE MAY BE ENHANCED BY SEDATIVE-ANTIANXIETY DRUGS. |
| 参考文献 |
|
| 其他信息 |
Crystals (in aqueous DMF). (NTP, 1992)
Chemically, dantrolene is a hydantoin derivative, but does not exhibit antiepileptic activity like other hydantoin derivates such as phenytoin. Dantrolene is a Skeletal Muscle Relaxant. The physiologic effect of dantrolene is by means of Decreased Striated Muscle Contraction, and Decreased Striated Muscle Tone. Dantrolene is a muscle relaxant used for treatment of chronic spasticity that differs from other commonly used muscle relaxants in acting peripherally on muscle, rather than centrally on the spinal cord or brain. Dantrolene can cause acute liver injury which can be severe and even fatal. Dantrolene is a hydantoin derivative and direct-acting skeletal muscle relaxant. Dantrolene depresses excitation-contraction coupling in skeletal muscle by binding to the ryanodine receptor 1, and decreasing intracellular calcium concentration. Ryanodine receptors mediate the release of calcium from the sarcoplasmic reticulum, an essential step in muscle contraction. Skeletal muscle relaxant that acts by interfering with excitation-contraction coupling in the muscle fiber. It is used in spasticity and other neuromuscular abnormalities. Although the mechanism of action is probably not central, dantrolene is usually grouped with the central muscle relaxants. See also: Dantrolene Sodium (has salt form). Drug Indication For use, along with appropriate supportive measures, for the management of the fulminant hypermetabolism of skeletal muscle characteristic of malignant hyperthermia crises in patients of all ages. Also used preoperatively, and sometimes postoperatively, to prevent or attenuate the development of clinical and laboratory signs of malignant hyperthermia in individuals judged to be malignant hyperthermia susceptible. Mechanism of Action Dantrolene depresses excitation-contraction coupling in skeletal muscle by binding to the ryanodine receptor 1, and decreasing intracellular calcium concentration. Ryanodine receptors mediate the release of calcium from the sarcoplasmic reticulum, an essential step in muscle contraction. DANTROLENE /PRODUCES RELAXATION &/ REDUCES CONTRACTION OF SKELETAL MUSCLE BY DIRECT ACTION ON EXCITATION-CONTRACTION COUPLING, PERHAPS BY DECR AMT OF CALCIUM RELEASED FROM SARCOPLASMIC RETICULUM. ...IT DOES NOT IMPAIR POLYSYNAPTIC REFLEXES PREFERENTIALLY AS DO CENTRALLY ACTING MUSCLE RELAXANTS. DANTROLENE DIMINISHES FORCE OF ELECTRICALLY INDUCED TWITCHES...WITHOUT ALTERING MUSCLE ACTION POTENTIALS.../&/ REDUCES REFLEX MORE THAN VOLUNTARY CONTRACTION. .../IT/ DOES NOT AFFECT NEUROMUSCULAR TRANSMISSION, NOR...CHANGE ELECTRICAL POTENTIAL PROPERTIES OF SKELETAL MUSCLE MEMBRANES. IN PATIENTS WITH UPPER MOTONEURON LESIONS, SPASTICITY IS GENERALLY DIMINISHED...& FUNCTIONAL CAPACITY IS OFTEN IMPROVED. DANTROLENE & 5-HYDROXYDANTROLENE INHIBITED RAT MUSCLE CONTRACTION RESPONSES IN DOSE-DEPENDENT MANNER IN VIVO & IN VITRO. 5-HYDROXYDANTROLENE WAS LESS POTENT THAN DANTROLENE. DANTROLENE INHIBITS CALCIUM 2+ ION (CA2+) RELEASE FROM THE SARCOPLASMIC RETICULUM OF FROG MUSCLE. IN RAT DIAPHRAGM PREPN DANTROLENE HAD NO EFFECT ON CONTRACTURES INDUCED BY 2,4-DINITROPHENOL, BUT REDUCED SIGNIFICANTLY THE CONTRACTURE PRODUCED BY K+. THE MAJOR ACTION OF DANTROLENE APPEARS TO BE ON THE SARCOLEMMA, WHICH MAY BE THE SITE OF THE MALIGNANT HYPERPYREXIA ABNORMALITY. DANTROLENE ADDED TO PREPN OF VOLTAGE-CLAMPED MYELINATED FROG NERVE FIBERS SHIFTED THE POTENTIAL-DEPENDENT PARAMETERS DESCRIBING SODIUM ION (NA+) PERMEABILITY TOWARDS MORE NEGATIVE MEMBRANE POTENTIALS. APPARENTLY, A CHANGE IN THE NEGATIVE SURFACE CHARGE OF THE MEMBRANE IS INDUCED. Therapeutic Uses Muscle Relaxants, Central DANTROLENE PROVIDES SIGNIFICANT & SUSTAINED REDUCTION OF SPASTICITY & IMPROVES FUNCTIONAL CAPACITY FOR MAJORITY OF PARAPLEGIC & HEMIPLEGIC PATIENTS; CLONUS, MASS-REFLEX MOVEMENTS & ABNORMAL RESISTANCE TO PASSIVE STRETCH ARE REDUCED. ABOUT 1/2 OF PT WITH ATHETOID CEREBRAL PALSY OR MULTIPLE SCLEROSIS ARE...SUFFICIENTLY IMPROVED... .../ACTION HELPFUL/ FOR PRE- & POST OPERATIVE MANAGEMENT OF MALIGNANT HYPERTHERMIA. ...OF SOME BENEFIT IN PATIENTS WITH EXTERNAL SPHINCTER HYPERTONICITY WHO HAVE EXCESSIVE RESIDUAL URINE VOLUME & HIGH URETHRAL PRESSURE. /DANTROLENE SODIUM/ ...SHOULD BE ADMIN IV AS SOON AS SYNDROME OF MALIGNANT HYPERTHERMIA IS RECOGNIZED; ... NECESSARY FOR 1 TO 3 DAYS TO PREVENT RECURRENCE... /DANTROLENE SODIUM/ For more Therapeutic Uses (Complete) data for DANTROLENE (9 total), please visit the HSDB record page. Drug Warnings .../IT/ TENDS TO INDUCE GENERALIZED MUSCLE WEAKNESS THAT CAN BE DETRIMENTAL TO FUNCTIONAL IMPROVEMENT. ...PT SHOULD BE CAUTIONED AGAINST DRIVING OR PARTICIPATING IN HAZARDOUS OCCUPATIONS. ... DANTROLENE SHOULD BE USED WITH CAUTION IN PT WITH IMPAIRED PULMONARY FUNCTION OR SEVERE MYOCARDIAL DISEASE. DANTROLENE IS CONTRAINDICATED IN LIVER DISEASE...& WHEN GROSS POSTURAL ABNORMALITIES RESULT FROM ITS USE. IT SHOULD PROBABLY BE WITHHELD IN PEPTIC ULCER PT. DANTROLENE IS NOT INDICATED IN FIBROSITIS, RHEUMATOID SPONDYLITIS, BURSITIS, ARTHRITIS OR ACUTE MUSCLE SPASM OF LOCAL ORIGIN. .../IT/ SHOULD NOT BE GIVEN TO PATIENTS WITH AMYOTROPHIC LATERAL SCLEROSIS, FOR THESE INDIVIDUALS HAVE VERY LOW TOLERANCE TO MUSCLE WEAKNESS INDUCED BY DANTROLENE. ...HEPATOCELLULAR INJURY...HAS BEEN FATAL IN SOME CASES. RISK APPEARS TO BE GREATEST IN PATIENTS OVER 30 YEARS, ESP WOMEN OVER 35 YEARS, WHO HAVE RECEIVED MORE THAN 300 MG DAILY FOR 60 DAYS OR LONGER. ...ROUTINE BASELINE HEPATIC FUNCTION STUDIES SHOULD BE PERFORMED PRIOR TO THERAPY, & SGOT OR SGPT & ALKALINE PHOSPHATASE LEVELS SHOULD BE DETERMINED MONTHLY DURING THERAPY. /DANTROLENE SODIUM/ ALTHOUGH WEAKNESS MAY BE TRANSIENT OR MILD, ITS PERSISTENCE IN SOME AMBULATORY PT MAY COMPROMISE THERAPEUTIC BENEFIT. ...DIARRHEA THAT OCCURS IN SOME PT CAN USUALLY BE CONTROLLED BY MORE GRADUAL INCR IN DOSAGE, IT MAY NECESSITATE WITHDRAWAL OF DRUG. For more Drug Warnings (Complete) data for DANTROLENE (7 total), please visit the HSDB record page. Pharmacodynamics Dantrolene is classified as a direct-acting skeletal muscle relaxant. It is currently the only specific and effective treatment for malignant hyperthermia. In isolated nerve-muscle preparation, Dantrium has been shown to produce relaxation by affecting the contractile response of the muscle at a site beyond the myoneural junction. In skeletal muscle, Dantrium dissociates excitation-contraction coupling, probably by interfering with the release of Ca2+ from the sarcoplasmic reticulum. In the anesthetic-induced malignant hyperthermia syndrome, evidence points to an intrinsic abnormality of skeletal muscle tissue. In selected humans, it has been postulated that “triggering agents” (e.g.,general anesthetics and depolarizing neuromuscular blocking agents) produce a change within the cell which results in an elevated myoplasmic calcium. This elevated myoplasmic calcium activates acute cellular catabolic processes that cascade to the malignant hyperthermia crisis. It is hypothesized that addition of Dantrium to the “triggered” malignant hyperthermic muscle cell reestablishes a normal level of ionized calcium in the myoplasm. |
| 分子式 |
C14H10N4O5
|
|---|---|
| 分子量 |
314.253
|
| 精确质量 |
314.065
|
| CAS号 |
7261-97-4
|
| 相关CAS号 |
Dantrolene sodium hemiheptahydrate;24868-20-0;Dantrolene sodium;14663-23-1;Dantrolene-13C3;1185234-99-4
|
| PubChem CID |
6914273
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 密度 |
1.57 g/cm3
|
| 沸点 |
175-177ºC
|
| 熔点 |
279-280°C (lit.)
|
| 折射率 |
1.715
|
| LogP |
2.53
|
| tPSA |
120.73
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
23
|
| 分子复杂度/Complexity |
524
|
| 定义原子立体中心数目 |
0
|
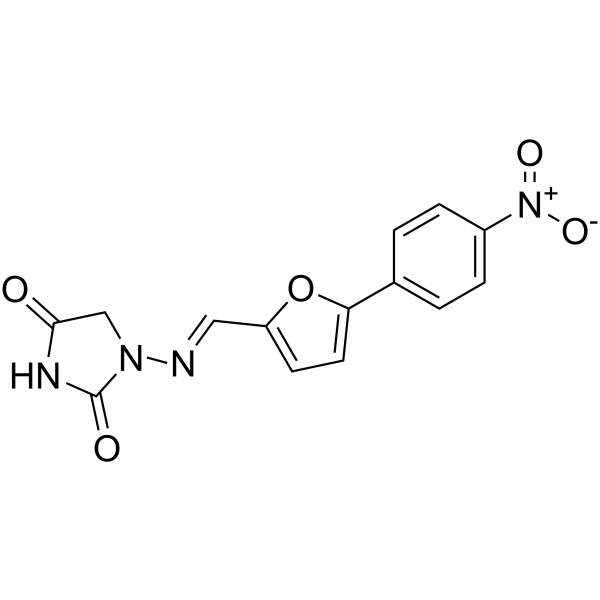
| SMILES |
C1C(=O)NC(=O)N1/N=C/C2=CC=C(O2)C3=CC=C(C=C3)[N+](=O)[O-]
|
| InChi Key |
OZOMQRBLCMDCEG-VIZOYTHASA-N
|
| InChi Code |
InChI=1S/C14H10N4O5/c19-13-8-17(14(20)16-13)15-7-11-5-6-12(23-11)9-1-3-10(4-2-9)18(21)22/h1-7H,8H2,(H,16,19,20)/b15-7+
|
| 化学名 |
1-[(E)-[5-(4-nitrophenyl)furan-2-yl]methylideneamino]imidazolidine-2,4-dione
|
| 别名 |
Dantrolenum; Dantroleno; Dantrolene
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 20 mg/mL (~63.64 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1822 mL | 15.9109 mL | 31.8218 mL | |
| 5 mM | 0.6364 mL | 3.1822 mL | 6.3644 mL | |
| 10 mM | 0.3182 mL | 1.5911 mL | 3.1822 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02829268 | Completed | Drug:dantrolene sodium | Wolfram Syndrome | Washington University School of Medicine |
January 2017 | Phase 1 Phase 2 |
| NCT04134845 | Active,not recruiting | Drug:Dantrolene/Ryanodex | Ventricular Tachycardia | Vanderbilt University Medical Center |
August 21, 2020 | Phase 2 Phase 3 |
| NCT03762109 | Recruiting | Drug:Dantrolene Drug:Placebo Oral Tablet |
Lumbar Spine Injury | Beth Israel Deaconess Medical Center |
July 29, 2019 | Phase 2 |
| NCT01024972 | Completed | Drug:Dantrolene Drug:Placebo |
Subarachnoid Hemorrhage | University of Massachusetts, Worcester | October 2009 | Phase 1 Phase 2 |
| NCT03109288 | Recruiting | Drug:Dantrolene Drug:Pirfenidone |
Multiple Sclerosis | National Institute of Allergy and Infectious Diseases (NIAID) |
August 11, 2017 | Phase 1 Phase 2 |
|
|