| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 体外研究 (In Vitro) |
在 ATCC 25923 和 E19977 中,双氯西林 EC50 值分别为 0.06 和 0.50 mg/L。在 pH 7.4 时,ATCC 25923 和 E19977 中双氯西林的最低抑制浓度分别为 0.125 和 0.5 mg/L [2]。
|
|---|---|
| 体内研究 (In Vivo) |
在小鼠腹膜炎脓毒症模型中,双氯西林具有治疗效果,所有小鼠均顺利渡过难关[3]。
|
| 动物实验 |
Animal/Disease Models: Female outbred Swiss Webster mice (murine peritonitis sepsis model) [3].
Doses: 125 mg/kg. Mode of Route of Administration: intravenous (iv) (iv)injection, single dose. Experimental Results: All mice survived. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorption of the isoxazolyl penicillins after oral administration is rapid but incomplete: peak blood levels are achieved in 1-1.5 hours. Oral absorption of cloxacillin, dicloxacillin, oxacillin and nafcillin is delayed when the drugs are administered after meals. Dicloxacillin sodium is rapidly excreted as unchanged drug in the urine by glomerular filtration and active tubular secretion. Differences in the elimination, distribution, and absorption of dicloxacillin and cloxacillin were studied in a group of healthy individuals with the use of a 2-compartment model. In patients on chronic intermittent hemodialysis, only dicloxacillin was investigated and the results were compared with data obtained in earlier studies on cloxacillin and flucloxacillin. In healthy volunteers the bioavailability after oral administration of 2 g dicloxacillin or 2 g cloxacillin amounted to 48.8% and 36.9% of the dose, respectively, when calculated from the area under the serum concentration-time curve, and to 74.1% and 48.5%, respectively, when calculated from the urinary excretion. Individual variation in bioavailability after oral administration was slightly lower for docloxacillin than for cloxacillin. The higher serum concentrations of dicloxacillin, as compared with cloxacillin, are also attributable to slower (renal) elimination (T 1/2: 42 and 33 min, respectively). Analysis of serum concentrations after intravenous administration of 1 and 2 g dicloxacillin to healthy subjects revealed concentration-dependent kinetics with respect ot renal elimination. In hemodialysis patients the elimination rate of dicloxacillin (T 1/2: 129 min) corresponds with the extrarenal elimination rate in healthy subjects. The bioavailability after oral administration of 1 g in patients is good (75.9% of the dose). Dicloxacillin, a semisynthetic isoxazolyl penicillin antibiotic, has antimicrobial activity against a wide variety of gram-positive bacteria including Staphylococcus aureus, Streptococcus pyogenes, Streptococcus pneumonia, Streptococcus epidermidis, Streptococcus viridans, Streptococcus agalactiae, and Neisseria meningitidis. The objective of this study was to evaluate the safety and pharmacokinetic profile of dicloxacillin after single and multiple oral dose in healthy Chinese volunteers. A single-center, open-label, randomized, two-phase study was conducted in 16 subjects. In the single-dose phase, subjects were randomly assigned to receive single doses of 0.25, 0.5, 1.0, and 2.0 g of dicloxacillin sodium capsule in a 4-way crossover design with a 5-day washout period between administrations. In the multiple-dose phase, subjects were assigned to receive 0.25 or 0.5 g every 6 hours for 3 days in a 2-way crossover design. Plasma and urine pharmacokinetic samples were assayed by a validated high-performance liquid chromatography-tandem mass spectrometry method. Pharmacokinetic parameters were calculated and analyzed statistically. Safety assessments were conducted throughout the study. Following a single oral dose of 0.25-2.0 g dicloxacillin sodium, the maximum plasma drug concentration (Cmax) and the corresponding values for the area under the concentration- time curve from 0 to 10 hours (AUC0-10 hr) increased in a dose-proportional manner. The mean elimination half-life (t1/2) was in the range of 1.38-1.71 hours. Dicloxacillin was excreted in its unchanged form via the kidney, with no tendency of accumulation, and varied from 38.65% to 50.10%. No appreciable accumulation of drug occurred with multiple oral doses of dicloxacillin. No serious adverse events were reported. Adverse events were generally mild. Dicloxacillin was safe and well tolerated in the volunteers and displayed linear increases in the Cmax and AUC0-10 hr values. The purpose of antibiotic treatment in pregnant women is to treat the mother and/or the fetus since it is known that antibiotics administered to the mother cross the placenta and reach the fetus. A comparison of the drug concentration in maternal and fetal plasma gives an indication of the exposure of the fetus to the maternally administered antibiotics. The aim of this study was to review the literature pertaining to the placental transfer of antibiotics in man and to classify the antibiotics according to the type of transfer involved ... 3 types of placental transfers were identified. A few antibiotics cross the placenta rapidly and equilibrate in the maternal and cord plasma; this type of transfer is termed "complete" and include the antibiotics ampicillin, methicillin, cefmenoxime and cefotiam. Antibiotics which show incomplete transfer to the placenta where concentrations are lower in the cord than maternal plasma are said to have "incomplete" transfer and these include azlocillin, dicloxacillin, piperacillin, sulbenicillin, cefoxitin, amikacin, gentamicin, kanamycin, streptomycin, fosfomycin, thiamphenicol, griseofulvin, vancomycin and colistimethate. ... All examined antibiotics cross the human placenta including those with a molecular weight greater than 1000 kDa such as vancomycin and colistimethate but there are 3 distinct types of placental transfer: complete, incomplete and exceeding and most antibiotics exhibit incomplete transfer. /The objective of the study was/ to determine whether upregulation of P-glycoprotein is responsible for the enhanced renal clearance of dicloxacillin in patients with cystic fibrosis ... Eleven patients with cystic fibrosis and 11 age-matched healthy volunteers /were used/. All subjects received a single oral dose of dicloxacillin 500 mg alone, dicloxacillin 500 mg plus probenecid (an organic anion transport inhibitor) 1 g, and dicloxacillin 500 mg plus cyclosporine (a P-glycoprotein inhibitor) 5 mg/kg; each treatment was separated by a washout period of 48 hours. A bolus dose of iothalamate meglumine 456 mg was administered on each study day as a marker of glomerular filtration. Blood and urine samples were taken serially up to 6 hours after each dose. Pharmacokinetics of dicloxacillin and iothalamate were determined by using compartmental and noncompartmental methods. Quantitative polymerase chain reaction was performed on peripheral blood mononuclear cells to measure expression of multidrug resistance 1 (MDR1) messenger RNA (mRNA). Genotyping for ABCB1 was performed to determine the presence of single nucleotide polymorphisms (exons 21 and 26). In both healthy subjects and patients with cystic fibrosis, compared with dicloxacillin alone, coadministration with probenecid produced a significantly lower renal clearance of dicloxacillin, whereas coadministration with cyclosporine resulted in no significant change; renal clearance was not significantly different between the two study groups. No correlation was found between MDR1 mRNA expression and renal clearance of dicloxacillin. The renal excretion of dicloxacillin was significantly greater in subjects with the ABCB1 exon 26 TT polymorphism when compared with subjects with the CT genotype. We found no significant difference in the pharmacokinetics of dicloxacillin between patients with cystic fibrosis and healthy volunteers. Renal clearance of dicloxacillin was significantly reduced in the presence of probenecid but not with cyclosporine, suggesting that the rate-limiting step in tubular secretion of dicloxacillin is uptake mediated by the organic anion transporter, and not P-glycoprotein inhibition. For more Absorption, Distribution and Excretion (Complete) data for Dicloxacillin (22 total), please visit the HSDB record page. Metabolism / Metabolites Dicloxacillin is partially metabolized to active and inactive metabolites. In one study following administration of a single 500-mg oral dose of dicloxacillin, 10% of the absorbed drug was hydrolyzed to penicilloic acids which are microbiologically inactive. Dicloxacillin is also hydroxylated to a small extent to a microbiologically active metabolite which appears to be slightly less active than dicloxacillin. Biological Half-Life The elimination half-life for dicloxacillin is about 0.7 hour. ... In hemodialysis patients the elimination rate of dicloxacillin (T 1/2: 129 min) corresponds with the extrarenal elimination rate in healthy subjects ... ... Following a single oral dose of 0.25-2.0 g dicloxacillin sodium, the maximum plasma drug concentration (Cmax) and the corresponding values for the area under the concentration- time curve from 0 to 10 hours (AUC0-10 hr) increased in a dose-proportional manner. The mean elimination half-life (t1/2) was in the range of 1.38-1.71 hours ... The serum half-life of dicloxacillin in adults with normal renal function is 0.6-0.8 hours. In one study in children 2-16 years of age, the serum half-life of the drug averaged 1.9 hours. The serum half-life of dicloxacillin is slightly prolonged in patients with impaired renal function and has been reported to range from 1-2.2 hours in patients with severe renal impairment. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Dicloxacillin therapy has not been associated with serum enzyme elevations during treatment, but has been linked to rare instances of clinically apparent, cholestatic hepatitis. The typical time to onset is 1 to 6 weeks and the pattern of serum enzyme elevations is usually cholestatic, although cases with a mixed pattern have also been described (Case 1). The injury usually presents with jaundice and pruritus. Fever, rash and eosinophilia can occur, but are not prominent and autoantibodies are rarely detected. A similar pattern of injury occurs more frequently with flucloxacillin (also called floxacillin) and cloxacillin, two oral isoxazolyl penicillins similar in structure and activity to dicloxacillin, but never approved for use or available in the United States. Similar cholestatic hepatitis arising 1 to 6 weeks after starting therapy occurs with other penicillins. Likelihood score: B (highly likely but rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited information indicates that dicloxacillin levels in milk are very low and are not expected to cause adverse effects in breastfed infants. It is frequently used to treat mastitis in nursing mothers. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with penicillins, but these effects have not been adequately evaluated. Dicloxacillin is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Binds to serum protein, mainly albumin. |
| 参考文献 |
|
| 其他信息 |
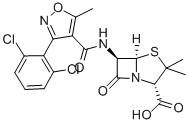
Dicloxacillin is a penicillin that is 6-aminopenicillanic acid in which one of the amino hydrogens is replaced by a 3-(2,6-dichlorophenyl)-5-methyl-1,2-oxazol-4-yl]formyl group. It has a role as an antibacterial drug. It is a penicillin and a dichlorobenzene. It is a conjugate acid of a dicloxacillin(1-).
One of the penicillins which is resistant to penicillinase. Dicloxacillin is a Penicillin-class Antibacterial. Dicloxacillin is an oral, second generation penicillin antibiotic that is used to treat bacterial infections caused by penicillinase-resistant staphylococci. Dicloxacillin has been linked to rare instances of clinically apparent, idiosyncratic liver injury. Dicloxacillin has been reported in Bos taurus with data available. Dicloxacillin is a broad-spectrum, semi-synthetic, beta-lactam, penicillin antibiotic with bactericidal and beta-lactamase resistant activity. Dicloxacillin binds to penicillin binding proteins (PBP) located on the inner membrane of the bacterial cell wall. It also inhibits the cross-linkage of peptidoglycan, a critical component of bacterial cell walls. This leads to the inhibition of bacterial cell wall synthesis and eventually causes cell lysis. One of the PENICILLINS which is resistant to PENICILLINASE. Drug Indication Used to treat infections caused by penicillinase-producing staphylococci which have demonstrated susceptibility to the drug. Mechanism of Action Dicloxacillin exerts a bactericidal action against penicillin-susceptible microorganisms during the state of active multiplication. All penicillins inhibit the biosynthesis of the bacterial cell wall. By binding to specific penicillin-binding proteins (PBPs) located inside the bacterial cell wall, dicloxacillin inhibits the third and last stage of bacterial cell wall synthesis. Cell lysis is then mediated by bacterial cell wall autolytic enzymes such as autolysins; it is possible that dicloxacillin interferes with an autolysin inhibitor. |
| 分子式 |
C19H17CL2N3O5S
|
|---|---|
| 分子量 |
470.33
|
| 精确质量 |
469.026
|
| CAS号 |
3116-76-5
|
| 相关CAS号 |
Dicloxacillin Sodium hydrate;13412-64-1;Dicloxacillin-13C4
|
| PubChem CID |
18381
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.6±0.1 g/cm3
|
| 沸点 |
692.4±55.0 °C at 760 mmHg
|
| 闪点 |
372.5±31.5 °C
|
| 蒸汽压 |
0.0±2.3 mmHg at 25°C
|
| 折射率 |
1.691
|
| LogP |
3.02
|
| tPSA |
138.04
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
746
|
| 定义原子立体中心数目 |
3
|
| SMILES |
CC1=C(C(N[C@@H]2C(N3[C@H](C(C)(S[C@H]23)C)C(O)=O)=O)=O)C(C4=C(Cl)C=CC=C4Cl)=NO1
|
| InChi Key |
YFAGHNZHGGCZAX-JKIFEVAISA-N
|
| InChi Code |
InChI=1S/C19H17Cl2N3O5S/c1-7-10(12(23-29-7)11-8(20)5-4-6-9(11)21)15(25)22-13-16(26)24-14(18(27)28)19(2,3)30-17(13)24/h4-6,13-14,17H,1-3H3,(H,22,25)(H,27,28)/t13-,14+,17-/m1/s1
|
| 化学名 |
(2S,5R,6R)-6-[[3-(2,6-dichlorophenyl)-5-methyl-1,2-oxazole-4-carbonyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
|
| 别名 |
Dicloxacilline Dicloxacilina Dicloxacillin
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1262 mL | 10.6308 mL | 21.2617 mL | |
| 5 mM | 0.4252 mL | 2.1262 mL | 4.2523 mL | |
| 10 mM | 0.2126 mL | 1.0631 mL | 2.1262 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。