| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
| 靶点 |
- DL-Homocysteine targets the brain kynurenic acid (a glutamate receptor antagonist) synthesis pathway [1]
- DL-Homocysteine (included in total homocysteine) targets pathways related to brain white matter integrity in Alzheimer’s disease [2] |
|---|---|
| 体外研究 (In Vitro) |
在大鼠皮质切片中,DL-同型半胱氨酸 (0.1-0.5 mM) 显着增加犬尿酸 (KYNA) 的合成,并在 3.0、5.0 和 10.0 mM 时抑制其产生,估计 IC50 为 6.4 (5.5-7.5) mM。在剂量≥0.2 mM时,DL-同型半胱氨酸剂量依赖性地抑制犬尿氨酸转氨酶 I (KATI) 的活性,IC50 为 0.566 (0.442-0.724) mM,抑制 KAT II 活性,IC50 值为 8.046 (5.804-11.154) mM。 1]。
- 大鼠脑冠状切片孵育实验:DL-同型半胱氨酸(DL-Homocysteine) 对体外犬尿氨酸合成呈双效作用。低浓度(10 μM)时促进犬尿氨酸生成,使其浓度较对照组增加约25%;高浓度(100、500 μM)时抑制合成,100 μM时浓度降低约30%,500 μM时降低约55%。采用高效液相色谱(HPLC)紫外检测(波长360 nm)测定犬尿氨酸含量[1] |
| 体内研究 (In Vivo) |
DL-同型半胱氨酸(1.3 mmol/kg,腹腔注射)将大鼠海马区的 KYNA 浓度(pmol/g 组织)从 4.11 ± 1.54 提高到 10.02 ± 3.08,将皮质中的 KYNA 浓度从 8.47 ± 1.57 提高到 13.04 ± 2.86 和 11.4 ± 1.72[1]。
- 人体阿尔茨海默病(AD)研究:45例AD患者根据血浆总同型半胱氨酸水平(含DL-同型半胱氨酸(DL-Homocysteine))分为高浓度组(>15 μmol/L)和正常浓度组(≤15 μmol/L),采用扩散张量成像(DTI)检测白质扩散参数。高浓度组额叶白质、胼胝体膝部的部分各向异性(FA)值较正常组分别降低约12%、15%,平均扩散率(MD)值分别升高约10%、13%,提示白质微结构损伤加重[2] |
| 酶活实验 |
- 脑犬尿氨酸转氨酶(KAT)活性实验:制备去除线粒体的大鼠脑匀浆,与含DL-同型半胱氨酸(DL-Homocysteine)(10、100、500 μM)及底物L-色氨酸(500 μM)的反应缓冲液混合,37°C孵育60分钟,加入三氯乙酸终止反应。离心后取上清,通过HPLC测定犬尿氨酸浓度,并根据犬尿氨酸生成量计算KAT活性。结果显示,10 μM DL-同型半胱氨酸(DL-Homocysteine)使KAT活性升高约20%,100 μM、500 μM时分别使KAT活性降低约28%、45%[1]
|
| 药代性质 (ADME/PK) |
Metabolism / Metabolites
In the body, dietary methionine is converted to homocysteine. In a series of metabolic steps, the enzyme cystathionine b-synthase (CBS) irreversibly generates a substance called cystathionine from homocysteine. The rate at which homocysteine is generated from methionine and then converted to cystathionine is evidently determined by the habitual dietary intake of methionine. L-Homocysteine has two primary fates: conversion via tetrahydrofolate (THF) back into L-methionine or conversion to L-cysteine. Homocysteine can cyclize to give homocysteine thiolactone, a five-membered heterocycle, a reaction catalyzed by methionyl-transfer RNA synthetase. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Nitrosylation converts homocysteine (Hcy) into a methionine analogue, S-nitroso-Homocysteine, which can substitute for methionine in protein synthesis in biological systems. In humans, homocyteine-thiolactone modifies proteins posttranslationally by forming adducts in which homocysteine is linked by amide bonds to epsilon-amino group of protein lysine residues (Hcy-epsilonN-Lys-protein). Levels of homocystine bound by amide or peptide linkages (Homocysteine-N-protein) in human plasma proteins are directly related to plasma 'total homocysteine' levels. Homocysteine-N-hemoglobin and Homocysteine-N-albumin constitute a major pool of homocysteine in human blood, larger than 'total homocysteine' pool. Homocysteine-thiolactone is present in human plasma. Modification with Homocysteine-thiolactone leads to protein damage and induces immune response. Autoantibodies that specifically recognize the Homocysteine-epsilonN-Lys-epitope on Homocysteine-thiolactone-modified proteins occur in humans. The ability of Homocysteine to interfere with protein biosynthesis, which causes protein damage, induces cell death and elicits immune response, is likely a key contributor to the toxicity of homocysteine (A15343). Uremic toxins such as homocysteine are actively transported into the kidneys via organic ion transporters (especially OAT3). Increased levels of uremic toxins can stimulate the production of reactive oxygen species. This seems to be mediated by the direct binding or inhibition by uremic toxins of the enzyme NADPH oxidase (especially NOX4 which is abundant in the kidneys and heart) (A7868). Reactive oxygen species can induce several different DNA methyltransferases (DNMTs) which are involved in the silencing of a protein known as KLOTHO. KLOTHO has been identified as having important roles in anti-aging, mineral metabolism, and vitamin D metabolism. A number of studies have indicated that KLOTHO mRNA and protein levels are reduced during acute or chronic kidney diseases in response to high local levels of reactive oxygen species (A7869). |
| 参考文献 | |
| 其他信息 |
Homocysteine is a sulfur-containing amino acid consisting of a glycine core with a 2-mercaptoethyl side-chain. It has a role as a fundamental metabolite. It is a sulfur-containing amino acid, a member of homocysteines and a non-proteinogenic alpha-amino acid. It is a conjugate acid of a homocysteinate. It is a tautomer of a homocysteine zwitterion.
DL-Homocysteine has been reported in Arabidopsis thaliana and Saccharomyces cerevisiae with data available. Homocysteine is a uremic toxin. Uremic toxins can be subdivided into three major groups based upon their chemical and physical characteristics: 1) small, water-soluble, non-protein-bound compounds, such as urea; 2) small, lipid-soluble and/or protein-bound compounds, such as the phenols and 3) larger so-called middle-molecules, such as beta2-microglobulin. Chronic exposure of uremic toxins can lead to a number of conditions including renal damage, chronic kidney disease and cardiovascular disease. Homocysteine is a sulfur-containing amino acid that arises during methionine metabolism. Although its concentration in plasma is only about 10 micromolar (uM), even moderate hyperhomocysteinemia is associated with increased incidence of cardiovascular disease and Alzheimer's disease. Elevations in plasma homocysteine are commonly found as a result of vitamin deficiencies, polymorphisms of enzymes of methionine metabolism, and renal disease. Pyridoxal, folic acid, riboflavin, and Vitamin B(12) are all required for methionine metabolism, and deficiency of each of these vitamins result in elevated plasma homocysteine. A polymorphism of methylenetetrahydrofolate reductase (C677T), which is quite common in most populations with a homozygosity rate of 10-15 %, is associated with moderate hyperhomocysteinemia, especially in the context of marginal folate intake. Plasma homocysteine is inversely related to plasma creatinine in patients with renal disease. This is due to an impairment in homocysteine removal in renal disease. Homocysteine is an independent cardiovascular disease (CVD) risk factor modifiable by nutrition and possibly exercise. Homocysteine was first identified as an important biological compound in 1932 and linked with human disease in 1962 when elevated urinary homocysteine levels were found in children with mental retardation. This condition, called homocysteinuria, was later associated with premature occlusive CVD, even in children. These observations led to research investigating the relationship of elevated homocysteine levels and CVD in a wide variety of populations including middle age and elderly men and women with and without traditional risk factors for CVD. (A3281, A3282). A thiol-containing amino acid formed by a demethylation of METHIONINE. - The dual effect of DL-Homocysteine on brain kynurenic acid synthesis is likely mediated by concentration-dependent regulation of kynurenine aminotransferase (KAT) activity—low concentrations activate KAT, while high concentrations inhibit it—thus altering the level of the glutamate receptor antagonist kynurenic acid [1] - Elevated plasma DL-Homocysteine (as part of total homocysteine) may exacerbate white matter microstructural damage in Alzheimer’s disease patients by enhancing oxidative stress and inflammatory responses, which is associated with disease progression [2] |
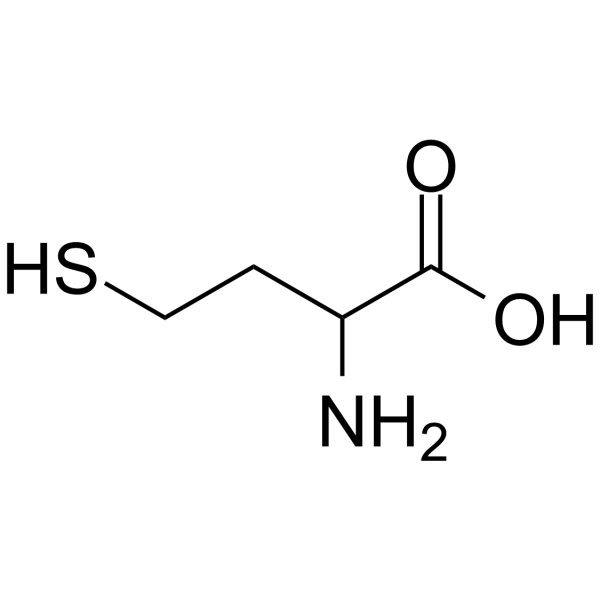
| 分子式 |
C4H9NO2S
|
|---|---|
| 分子量 |
135.18476
|
| 精确质量 |
135.035
|
| CAS号 |
454-29-5
|
| PubChem CID |
778
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
299.7±35.0 °C at 760 mmHg
|
| 熔点 |
232-233 °C(lit.)
|
| 闪点 |
135.0±25.9 °C
|
| 蒸汽压 |
0.0±1.3 mmHg at 25°C
|
| 折射率 |
1.538
|
| LogP |
0.22
|
| tPSA |
102.12
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
8
|
| 分子复杂度/Complexity |
86.1
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C(O)C(N)CCS
|
| InChi Key |
FFFHZYDWPBMWHY-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C4H9NO2S/c5-3(1-2-8)4(6)7/h3,8H,1-2,5H2,(H,6,7)
|
| 化学名 |
2-amino-4-sulfanylbutanoic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~125 mg/mL (~924.62 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.3975 mL | 36.9877 mL | 73.9754 mL | |
| 5 mM | 1.4795 mL | 7.3975 mL | 14.7951 mL | |
| 10 mM | 0.7398 mL | 3.6988 mL | 7.3975 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。