| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 靶点 |
Aromatase; ERα
|
|---|---|
| 体外研究 (In Vitro) |
Endoxifen 是一种羟基化他莫昔芬代谢物,作为 ER 拮抗剂,其效果比他莫昔芬高约 100 倍。这还意味着,除了对 ER 的转录相关影响之外,内昔芬(而非 4-羟基他莫昔芬)还会导致 ER-α 降解[1]。 Endoxifen 是一种强效抗雌激素,会导致乳腺癌细胞的雌激素受体 α 降解。此外,研究表明,即使存在他莫昔芬、N-去甲基他莫昔芬和 4-羟基他莫昔芬,endoxifen 也会抑制雌激素诱导的乳腺癌细胞增殖并阻断 ERA 转录活性[2]。在 10 μM 浓度下,多昔芬显着抑制所有乳腺癌细胞系的生长,但 MDAMB-468 除外,它仅受到中度抑制。在 10 μM 浓度下,对 MCF7、HS 578T 和 BT-549 细胞的细胞毒性作用相当明显。重要的。虽然 Endoxifen 在较低浓度 (0.01-1 μM) 下的抑制作用不如 10 μM 强,但所有测试细胞在浓度为 100 μM 时都会死亡[2]。
|
| 体内研究 (In Vivo) |
在雌性大鼠身上进行测试时,口服的内多昔芬很快就会被吸收并遍布全身。与他莫昔芬治疗的大鼠相比,用内多昔芬治疗的大鼠表现出高 1,500% 的 Cmax 和高 787% 的内多昔芬暴露 (AUC0–∞) 水平。口服恩多昔芬(Endoxifen)剂量为2、4和8 mg/kg,每天一次,连续28天是安全的,并且会导致雌性小鼠体内人乳腺肿瘤异种移植物的生长逐渐受到抑制[2]。
|
| 动物实验 |
Mice: A subcutaneous (s.c.) implant of a 30-to 40-mg fragment of MCF-7 human mammary tumor from an in vivo passage is made in six-week-old female athymic NCr-nu/nu mice near the right flank. Day 0 is the date of the implantation of the tumor fragments. Each animal has a 0.72-mg 17 β-estradiol 60-day release pellet s.c. implanted in the back of their neck one day before the tumor fragment is inserted, in order to support the growth of the estrogen-dependent MCF-7 tumor. Day 13 following tumor fragment implantation, the day treatment was started, saw the growth of individual tumors to sizes ranging from 75 to 196 mm3. One control group (12 mice/group) and four treatment groups (6 mice/group) are created from a total of thirty-six tumor-bearing mice through randomization. On day 13 after the tumor was implanted, the patient was given oral gavage once a day for 28 days, along with three different doses of Endoxifen (2, 4, and 8 mg/kg) or Tamoxifen (10 mg/kg) twice a day, three hours apart. For every treatment group, the dosage volume of 0.2 mL/10 g body weight remains unchanged. Beginning on the first day of treatment, the s.c. tumors are measured and the animals are weighed twice a week. On Day 58, the research comes to an end. The overall delay in the growth of the median tumor is determined by taking the median time to reach two tumor mass doublings. Furthermore, an additional assessment of the antitumor efficacy is conducted on Days 41, 1 day after the last treatment, and 58, the day the study is terminated, by comparing the median tumor weight in the treatment groups to the median tumor weight in the control group (T/C 9 100%)[2].
|
| 药代性质 (ADME/PK) |
Metabolism / Metabolites
Endoxifen has known human metabolites that include Endoxifen O-glucuronide and Endoxifen O-sulfate. Endoxifen is a known human metabolite of 4-Hydroxytamoxifen and N-Desmethyltamoxifen. |
| 参考文献 |
|
| 其他信息 |
4-Hydroxy-N-desmethyltamoxifen is a stilbenoid.
|
| 分子式 |
C₂₅H₂₇NO₂
|
|---|---|
| 分子量 |
373.48738
|
| 精确质量 |
373.204
|
| CAS号 |
110025-28-0
|
| 相关CAS号 |
Endoxifen (Z-isomer);112093-28-4;Endoxifen Z-isomer hydrochloride;1032008-74-4;Endoxifen hydrochloride;1197194-41-4;Endoxifen (E-isomer);114828-90-9;Endoxifen E-isomer hydrochloride;1197194-61-8;Endoxifen-d5;1185244-45-4
|
| PubChem CID |
10090750
|
| 外观&性状 |
White to light yellow solid
|
| 密度 |
1.099g/cm3
|
| 沸点 |
519.3ºC at 760mmHg
|
| 闪点 |
267.9ºC
|
| 蒸汽压 |
2.1E-11mmHg at 25°C
|
| 折射率 |
1.598
|
| LogP |
5.75
|
| tPSA |
41.49
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
467
|
| 定义原子立体中心数目 |
0
|
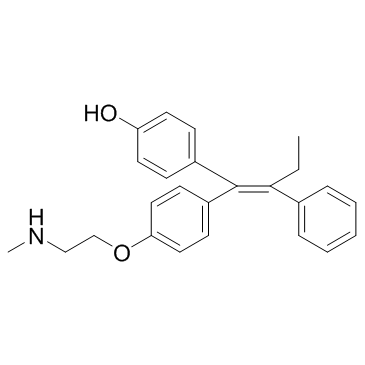
| SMILES |
CC/C(C1=CC=CC=C1)=C(C2=CC=C(O)C=C2)/C3=CC=C(OCCNC)C=C3
|
| InChi Key |
MHJBZVSGOZTKRH-OCOZRVBESA-N
|
| InChi Code |
InChI=1S/C25H27NO2/c1-3-24(19-7-5-4-6-8-19)25(20-9-13-22(27)14-10-20)21-11-15-23(16-12-21)28-18-17-26-2/h4-16,26-27H,3,17-18H2,1-2H3/b25-24+
|
| 化学名 |
4-[(E)-1-[4-[2-(methylamino)ethoxy]phenyl]-2-phenylbut-1-enyl]phenol
|
| 别名 |
Endoxifen
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6774 mL | 13.3872 mL | 26.7745 mL | |
| 5 mM | 0.5355 mL | 2.6774 mL | 5.3549 mL | |
| 10 mM | 0.2677 mL | 1.3387 mL | 2.6774 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04315792 | Recruiting | Drug: Endoxifen Drug: Placebo oral tablet |
Bipolar I Disorder | Jina Pharmaceuticals Inc. | March 23, 2020 | Phase 3 |
| NCT05607004 | Recruiting | Drug: exemestane Drug: (Z)-endoxifen |
Breast Neoplasms Invasive Breast Cancer |
Atossa Therapeutics, Inc. | February 14, 2023 | Phase 2 |
| NCT01273168 | Active Recruiting |
Drug: Z-Endoxifen | Gynecologic Desmoid |
National Cancer Institute (NCI) |
March 1, 2011 | Phase 1 |
| NCT01327781 | Active Recruiting |
Drug: Endoxifen Hydrochloride Other: Pharmacological Study |
HER2/Neu Positive Recurrent Breast Carcinoma |
National Cancer Institute (NCI) |
March 25, 2011 | Phase 1 |
| NCT05068388 | Recruiting | Drug: Z-Endoxifen Drug: Placebo |
Breast Density | Atossa Therapeutics, Inc. | December 21, 2021 | Phase 2 |