| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 靶点 |
Class A KPC-2 (IC50 = 4 nM); Class C AmpC (IC50 = 14 nM); D OXA-24 (IC50 = 190 nM)
|
|---|---|
| 体外研究 (In Vitro) |
Durlobactam是一种比其他DBOs阿维巴坦和雷巴坦更有效的BlaC抑制剂;Durlobactam是结核分枝杆菌几种肽聚糖转肽酶的有效抑制剂;Durlobactam恢复了M。结核分离出β-内酰胺类[3]
ETX2514是一种具有内在抗菌活性的抗氧化剂,可以增强其恢复碳青霉烯对CRE菌株的活性的能力。耐多药细菌感染是对公众健康的严重威胁。最令人担忧的耐药性趋势之一是β-内酰胺酶的数量和多样性的迅速增加,β-内胺酶是一类使β-内酰胺失活的酶,几十年来一直是治疗的支柱。尽管一些新的β-内酰胺酶抑制剂已经被批准或正在进行临床试验,但它们的活性谱并不能解决多药耐药病原体,如鲍曼不动杆菌。本报告描述了扩谱丝氨酸β-内酰胺酶抑制剂的合理设计和特性,该抑制剂能有效抑制临床相关的A、C和D类β-内酶以及青霉素结合蛋白,从而对肠杆菌科产生内在抗菌活性,并在广泛的耐多药革兰阴性病原体中恢复β-内内酰胺活性。舒巴坦-ETX2514是最有前景的组合之一,其强大的抗菌活性、对耐多药鲍曼菌感染的体内疗效和有希望的临床前安全性证明了其解决这一重大未满足医疗需求的潜力[1] 在棋盘格分析中,对β-内酰胺/β-内内酰胺酶抑制剂硬洛巴坦和17种抗菌剂的组合对鲍曼不动杆菌菌株进行了测试。大多数组合导致冷漠,没有对抗的情况。这些结果表明,如果与其他抗菌药物联合给药,硬洛巴坦对鲍曼不动杆菌的抗菌活性不太可能受到影响[2]。 |
| 体内研究 (In Vivo) |
舒巴坦–ETX2514在耐多药鲍曼氏杆菌感染小鼠模型中显示出体内疗效[1]
单用舒巴坦的体内中性粒细胞减少性肺和大腿感染模型研究[4] 在单用舒巴坦与鲍曼不动杆菌ARC2058进行的大腿研究中,与1-log10 CFU/g减少、2-log10 CFU/g减少和EC80相关的%fT>MIC幅度分别为20.5、31.5和47.0(表3)。在肺模型中,与1-log10 CFU/g减少、2-log10 CFU/g减少和EC80相关的平均%fT>MIC幅度分别为37.8、50.1和68.5 舒巴坦联合硬洛巴坦与CRAB菌株的体内中性粒细胞减少性大腿和肺部感染模型研究[4] 表3总结了舒巴坦用于大腿和肺部感染模型的单个菌株%fT>MIC估计值,以实现1-log10 CFU/g减少、2-log10 CFU/g减少的PK/PD终点,以及EC80与CRAB菌株的比较。多个CRAB菌株和舒巴坦易感菌株ARC2058的%fT>MIC舒巴坦暴露反应数据(与杜洛巴坦联合给药时)的联合建模如图所示。1用于大腿和肺模型。表3总结了与1-log10 CFU/g减少、2-log10 CFU/g减少和联合建模数据的EC80相关的舒巴坦%fT>MIC幅度。1-log10需要%fT>MIC的幅度,与通过数据联合建模确定的PK/PD终点相比,在所有菌株中确定的单个PK/PD端点的平均值之间,2-log10 CFU的减少几乎相同 硬洛巴坦是一种β-内酰胺/β-内酶抑制剂组合,目前正在开发中,用于治疗不动杆菌引起的感染,包括耐多药(MDR)分离株。尽管舒巴坦是Ambler a类酶亚群的β-内酰胺酶抑制剂,但它也对包括不动杆菌在内的有限数量的细菌表现出固有的抗菌活性,并已有效用于治疗易感不动杆菌相关感染。然而,越来越多的β-内酰胺酶介导的耐药性已经削弱了舒巴坦治疗这种病原体的有效性。杜拉巴坦是一种设计合理的二氮杂双环辛烷类β-内酰胺酶抑制剂。该化合物表现出对丝氨酸β-内酰胺酶活性的广谱抑制作用,对D类酶具有特别强的活性,这是它与其他DBO抑制剂的区别。当与舒巴坦联合使用时,杜洛巴坦通过抑制β-内酰胺酶有效地恢复耐药菌株的易感性。本综述描述了在多药耐药鲍曼不动杆菌分离株的非临床感染模型中建立的与舒巴坦和硬洛巴坦活性相关的药代动力学/药效学(PK/PD)关系。这些信息有助于确定PK/PD的疗效靶点,可用于预测联合用药在人类中的有效剂量方案。[5] |
| 酶活实验 |
PonA1和Ldts[3]的抑制动力学
方程1的反应方案和Henri–Michaelis–Menten方程(方程2)也用于表征PonA1和Mtb的Ldts的抑制动力学值。使用BioTek Synergy2多模微孔板读取器和Gen5分析软件在30°C下使用0.25 cm路径进行纯化酶的动力学测定。反应在50mM Tris-HCl(pH 7.5)和300mM氯化钠中进行,并且硝基烯烃类是所有测定的报告底物。为了获得每种酶的KmNCF,随着硝基烯浓度的增加,对固定浓度的酶在482 nm的λ处15分钟内的吸光度变化进行监测。使用Origin 8.1通过非线性最小二乘法拟合方程2来拟合数据。随后,使用硝酸烯浓度为5×KmNCF的固定浓度的酶和增加的抑制剂浓度,测定每种酶-抑制剂组合的KI-app。在30分钟内测量每个反应在482 nm的λ处的吸光度变化。使用方程3和BlaC测定1/Δ吸光度与[抑制剂]线性化数据和校正的KI-app的Dixon图 BlaC[3]的抑制动力学 如前所述,用纯化的BlaC酶测定抑制动力学。简言之,反应方案如等式1所示,其中E是酶,S是底物,E:S是Michaelis–Menten复合物,E–S是酰基酶复合物,P是反应产物。使用安捷伦8453二极管阵列分光光度计测定BlaC的动力学参数)。在室温下,在1cm路径长度的比色皿中在100mM吗啉乙磺酸(MES)(pH 6.4)中进行反应。首先,硝基烯烃的参数(Δε=17 400 M–1 cm–1)经BlaC水解。KmNCF,硝基烯的米氏常数是硝基烯的浓度[S],其中反应速率v等于最大反应速率Vmax的一半。测量了与0.28μg/mL BlaC混合的硝基烯烃浓度增加时的初始反应速率,并根据等式2使用Origin 8.1对数据(Henri–Michaelis–Menten方程)进行非线性最小二乘拟合。。。 |
| 细胞实验 |
体外药敏试验[3]
ATCC结核分枝杆菌H37Ra、H37Rv和9个当代临床结核分枝杆菌分离株通过肉汤微量稀释进行抗菌药物敏感性测试。抗生素化合物是从商业来源购买的,但由Entasis Therapeutics慷慨提供的杜洛巴坦除外。Middlebrook 7H9肉汤补充有10%(v/v)油酸白蛋白-葡萄糖-过氧化氢酶(OADC)、0.05%(v/vTween 80和0.5%(v/v甘油)作为培养基。使用96孔微孔板对药物进行连续2倍稀释。对于组合,添加固定浓度为2.5μg/mL的棒酸盐;所有其他组合(β-内酰胺/杜洛巴坦或双β-内胺)以1:1质量/体积比组合。用约5×105菌落形成单位(CFU)/mL接种微孔板孔。在37°C下培养14-18天后,阻止可见生长的最低抗生素浓度记录为MIC。用基于雷沙祖林的试剂alamarBlue HS确认可见生长。 |
| 动物实验 |
In vivo neutropenic lung and thigh infection models [4]
In vivo infection models of pneumonia and thigh tissue abscess were performed in CD-1 mice (15–18 g) rendered neutropenic by cyclophosphamide treatment prior to infection as previously described. In the lung model, mice were infected with A. baumannii isolates in 1% agar slurry via direct intratracheal instillation. In the thigh model, mice were infected intramuscularly in the right thigh. Infected mice were treated with either sulbactam alone or sulbactam combined with durlobactam at a constant 4:1 ratio. Dosing was initiated 2 hours after infection and administered as eight subcutaneous (SC) injections administered q3h or four SC injections administered q6h. Vehicle groups received a single SC injection, and positive control groups received either a single SC injection of colistin sulphate (maximum tolerated dose of 40 mg/kg) or two oral doses of levofloxacin (200 mg/kg bid) 2 hours after infection. Colistin sulphate administered as a single 40-mg/kg dose achieved 0.25 to 2.6 log10 CFU/g reduction vs. MDR A. baumannii strains, and levofloxacin achieved −2.4 log10 CFU vs. A. baumannii ARC2058 in the thigh model. In the lung model, colistin administration resulted in 1-log10 CFU/g growth to 2.1 log10 CFU/g reduction vs. MDR A. baumannii strains and levofloxacin administration resulted in 1.8 log10 CFU/g reduction vs. A. baumannii ARC2058. The overall performance of positive controls was acceptable relative to isolate susceptibility. Efficacy was determined by harvesting infected tissue and determining viable bacterial counts 24 hours after the start of treatment. A full dose response study utilized meropenem to validate the model system, where meropenem was administered SC on a q6h regimen from 1 to 300 mg/kg following uranyl nitrate pre-treatment to extend drug exposure. Subcutaneous doses of sulbactam and durlobactam were administered at a 4:1 ratio at doses of 20:5, 40:10, 60:15, 80:20, and 160:40 mg/kg (SUL:DUR) with plasma PK sampling (n = 3 samples/timepoint) at 0.5, 1, 2, 3, 4, 6, 8, 10, and 12 hours post dose. These studies were completed in neutropenic CD-1 mice with active thigh infections. An additional PK and ELF distribution study was completed at a SUL:DUR dose of 100:25 mg/kg in neutropenic lung-infected animals with PK timepoints sampled at 0, 1, 3, 6, and 12 hours post dose. Blood was processed for plasma using microcontainer tubes containing ethylenediamine tetraacetic acid (EDTA, Beckton Dickenson) and centrifugation for 5 minutes at 13,200 rpm. Plasma samples were treated 1:1 with a SigmaFAST protease inhibitor cocktail prepared from one tablet dissolved in 10 mL of deionized water. The samples were then stored at −80°C until bioanalysis. Pharmacokinetic-pharmacodynamic analysis[4] As the plasma PK of neutropenic lung-infected animals matched that of thigh-infected animals, the thigh-infected PK was used for the development of the population PK model and subsequent exposure estimates for doses used in the PK/PD exposure-response analyses. Pharmacokinetic models were fit to time vs. drug concentration profiles generated in infected mice using Phoenix6.2 WinNonLin and NLME. For the purposes of estimating exposures across all doses used in dose response studies, population PK models were developed for sulbactam alone, for sulbactam when co-administered with durlobactam, and for durlobactam co-administered with sulbactam (dose ratio 4:1, sulbactam:durlobactam). Population PK parameter estimates were derived from a two-compartment PK model incorporating a log-additive error model constructed from fitting of sulbactam and durlobactam concentration vs. time data (Table S4). Representative model fit concentration vs. time profiles vs. observed data are shown in Fig. S1 for sulbactam at 320 and 40 mg/kg and durlobactam at 80 and 10 mg/kg. Predicted exposures were utilized in the PK/PD analyses as all dosing solutions tested were within the variability of the bioanalytical method (20%). For pharmacokinetic-pharmacodynamic evaluation sulbactam %fT>MIC and durlobactam fAUC0-24/MIC were simulated for each dose using the population PK model. A Hill-type model was fit to the pharmacodynamic data generated from the dose response studies with linear least squares regression analysis of the relationships between change in log10 CFU from baseline at 24 hours and sulbactam %fT>MIC and durlobactam fAUC/MIC. Magnitudes associated with 1-log10 and 2-log10 CFU reduction from baseline at 24 hours as well as the exposure required for an 80% reduction in bacterial burden (EC80) were determined from the fitted function. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Sulbactam produces low levels in milk that are not expected to cause adverse effects in breastfed infants. It is likely that durlobactam produces similar levels in milk. Occasionally, disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush, have been reported with penicillins, but these effects have not been adequately evaluated. Sulbactam-durlobactam is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| 参考文献 |
[2] Plasma and Intrapulmonary Concentrations of ETX2514 and Sulbactam following Intravenous Administration of ETX2514SUL to Healthy Adult Subjects. Antimicrob Agents Chemother. 2018 Aug 20. pii: AAC.01089-18. [2]. In vitro antibacterial activity of sulbactam-durlobactam in combination with other antimicrobial agents against Acinetobacter baumannii-calcoaceticus complex. Diagn Microbiol Infect Dis . 2024 May 9;109(3):116344.[3]. Durlobactam, a Diazabicyclooctane β-Lactamase Inhibitor, Inhibits BlaC and Peptidoglycan Transpeptidases of Mycobacterium tubercul. ACS Infect Dis . 2024 May 10;10(5):1767-1779. [4]. In vivo dose response and efficacy of the β-lactamase inhibitor, durlobactam, in combination with sulbactam against the Acinetobacter baumannii-calcoaceticus complex. Antimicrob Agents Chemother. 2024 Jan; 68(1): e00800-23. [5]. he Pharmacokinetics/Pharmacodynamic Relationship of Durlobactam in Combination With Sulbactam in In Vitro and In Vivo Infection Model Systems Versus Acinetobacter baumannii-calcoaceticus Complex. Clin Infect Dis . 2023 May 1;76(Suppl 2):S202-S209. |
| 其他信息 |
Durlobactam sodium is an organic sodium salt that is the monosodium salt of durlobactam. It has a role as an EC 3.5.2.6 (beta-lactamase) inhibitor and an antibacterial drug. It contains a durlobactam(1-).
See also: Durlobactam (has active moiety) ... View More ... |
| 分子式 |
C8H10N3NAO6S
|
|---|---|
| 分子量 |
299.2363
|
| 精确质量 |
277.04
|
| 元素分析 |
C, 32.11; H, 3.37; N, 14.04; Na, 7.68; O, 32.08; S, 10.71
|
| CAS号 |
1467157-21-6
|
| 相关CAS号 |
1467829-71-5 (free acid);1467157-21-6 (sodium);
|
| PubChem CID |
89851851
|
| 外观&性状 |
White to light yellow solid
|
| tPSA |
141
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
19
|
| 分子复杂度/Complexity |
541
|
| 定义原子立体中心数目 |
2
|
| SMILES |
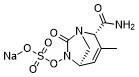
CC1=C[C@@H]2CN([C@@H]1C(=O)N)C(=O)N2OS(=O)(=O)[O-].[Na+]
|
| InChi Key |
WHHNOICWPZIYKI-IBTYICNHSA-M
|
| InChi Code |
InChI=1S/C8H11N3O6S.Na/c1-4-2-5-3-10(6(4)7(9)12)8(13)11(5)17-18(14,15)16/h2,5-6H,3H2,1H3,(H2,9,12)(H,14,15,16)/q+1/p-1/t5-,6+/m1./s1
|
| 化学名 |
sodium (2S,5R)-2-carbamoyl-3-methyl-7-oxo-1,6-diazabicyclo[3.2.1]oct-3-en-6-yl sulfate
|
| 别名 |
ETX2514 ETX-2514 ETX 2514 ETX2514 sodium Durlobactam sodium
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O: ~250 mg/mL (835 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3418 mL | 16.7090 mL | 33.4180 mL | |
| 5 mM | 0.6684 mL | 3.3418 mL | 6.6836 mL | |
| 10 mM | 0.3342 mL | 1.6709 mL | 3.3418 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。