| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
DA1 receptor ( EC50 = 55.5 nM )
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Radiolabeled studies show that about 90% of infused fenoldopam is eliminated in urine, 10% in feces. Elimination is largely by conjugation, without participation of cytochrome P-450 enzymes. Only 4% of the administered dose is excreted unchanged. Metabolism / Metabolites Elimination is largely by conjugation, without participation of cytochrome P-450 enzymes. Methylation, glucuronidation, and sulfation are the main routes of conjugation. Biological Half-Life The elimination half-life is about 5 minutes in mild to moderate hypertensives, with little difference between the R (active) and S isomers. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the use of fenoldopam during breastfeeding. The manufacturer recommends avoiding breastfeeding during fenoldopam use; however, because of its poor oral bioavailability and short half-life, any fenoldopam in milk is unlikely to adversely affect the breastfed infant. Also, fenoldopam can be given intravenously to infants. Unlike dopamine, it does not decrease serum prolactin concentrations and might not interfere with nursing. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information in nursing mothers was not found as of the revision date. Unlike dopamine, fenoldopam infusion does not affect serum prolactin concentration in normal women. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. |
| 参考文献 | |
| 其他信息 |
Fenoldopam is a benzazepine. It has a role as a dopaminergic antagonist, a vasodilator agent, an alpha-adrenergic agonist, a dopamine agonist and an antihypertensive agent.
A dopamine D1 receptor agonist that is used as an antihypertensive agent. It lowers blood pressure through arteriolar vasodilation. Fenoldopam is a Dopaminergic Agonist. The mechanism of action of fenoldopam is as a Dopamine Agonist. Fenoldopam is a benzazepine derivative with vasodilatory and antihypertensive properties. Fenoldopam, a dopamine (DA) receptor agonist, binds specifically to peripheral DA1 receptors and to alpha-2 adrenoceptors with moderate affinity. However, this agent exhibits no significant affinity to DA2, other alpha adrenergic, beta adrenergic, muscarinic, or serotonergic receptors. Receptor binding modulates the transmembrane flux of ions, thereby stimulating adenylate cyclase activity, as well as the release of prolactin. This results in vasodilatation, increased renal blood flow thereby enhancing natriuresis and diuresis leading to a lowering in diastolic blood pressure. A dopamine D1 receptor agonist that is used as an antihypertensive agent. It lowers blood pressure through arteriolar vasodilation. See also: Fenoldopam Mesylate (has salt form). Drug Indication For the in-hospital, short-term (up to 48 hours) management of severe hypertension when rapid, but quickly reversible, emergency reduction of blood pressure is clinically indicated, including malignant hypertension with deteriorating end-organ function. FDA Label Mechanism of Action Fenoldopam is a rapid-acting vasodilator. It is an agonist for D1-like dopamine receptors and binds with moderate affinity to α2-adrenoceptors. It has no significant affinity for D2-like receptors, α1 and β-adrenoceptors, 5HT1 and 5HT2 receptors, or muscarinic receptors. Fenoldopam is a racemic mixture with the R-isomer responsible for the biological activity. The R-isomer has approximately 250-fold higher affinity for D1-like receptors than does the S-isomer. In non-clinical studies, fenoldopam had no agonist effect on presynaptic D2-like dopamine receptors, or α or β -adrenoceptors, nor did it affect angiotensin-converting enzyme activity. Fenoldopam may increase norepinephrine plasma concentration. |
| 分子式 |
C16H16CLNO3
|
|---|---|
| 分子量 |
305.75614
|
| 精确质量 |
305.081
|
| CAS号 |
67227-56-9
|
| 相关CAS号 |
Fenoldopam mesylate; 67227-57-0; Fenoldopam hydrochloride; 181217-39-0
|
| PubChem CID |
3341
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
522.6±50.0 °C at 760 mmHg
|
| 闪点 |
269.9±30.1 °C
|
| 蒸汽压 |
0.0±1.4 mmHg at 25°C
|
| 折射率 |
1.656
|
| LogP |
1.72
|
| tPSA |
72.72
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
21
|
| 分子复杂度/Complexity |
348
|
| 定义原子立体中心数目 |
0
|
| SMILES |
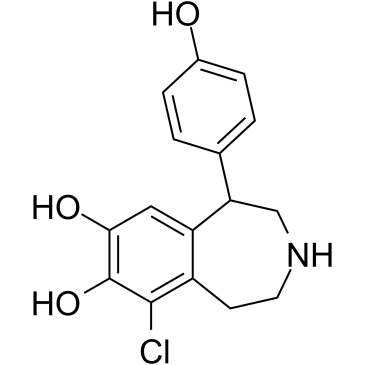
OC1=C(O)C=C2C(C3=CC=C(O)C=C3)CNCCC2=C1Cl
|
| InChi Key |
TVURRHSHRRELCG-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2
|
| 化学名 |
9-chloro-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro-1H-3-benzazepine-7,8-diol
|
| 别名 |
SKF 82526; Fenoldopam
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~77 mg/mL (~251.8 mM)
Water: ~10 mg/mL |
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.2705 mL | 16.3527 mL | 32.7054 mL | |
| 5 mM | 0.6541 mL | 3.2705 mL | 6.5411 mL | |
| 10 mM | 0.3271 mL | 1.6353 mL | 3.2705 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00621790 | Completed | Drug: fenoldopam Drug: placebo |
Acute Renal Failure | Università Vita-Salute San Raffaele |
February 2008 | Phase 3 |
| NCT00982527 | Completed | Drug: Placebo Drug: Fenoldopam |
Kidney Failure, Acute | Bambino Gesù Hospital and Research Institute |
September 2009 | Phase 3 |
| NCT00122018 | Completed | Drug: N-acetylcysteine Drug: fenoldopam |
Kidney Failure, Acute Kidney Failure, Chronic |
Linda F. Barr, M.D. | May 2002 | Phase 2 |
| NCT00747331 | Completed | Drug: Fenoldopam mesilate Drug: Placebo |
Cardiac Complications Cardiopulmonary Bypass |
IRCCS Policlinico S. Donato | September 2008 | Phase 4 |
| September 2008 | Completed | Drug: Fenoldopam | Salt-sensitive Hypertension | Georgetown University | November 2002 | Not Applicable |