| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|

| 靶点 |
type I PRMT; PRMT1 (IC50 = 3.1 nM); PRMT3 (IC50 = 48 nM); PRMT4 (IC50 = 1148 nM); PRMT6 (IC50 = 5.7 nM); PRMT1 (IC50 = 1.8 nM) ; Type I protein arginine methyltransferases (PRMT1, PRMT3, PRMT4, PRMT6, PRMT8)
|
|---|---|
| 体外研究 (In Vitro) |
GSK3368715 (EPZ019997) 是代表 12 种肿瘤类型的 249 种癌细胞系之一,与用 DMSO 处理的细胞相比,表现出至少 50% 的生长抑制 [1]。
- GSK3368715是一种强效、可逆且不与S-腺苷甲硫氨酸(SAM)竞争的I型PRMT抑制剂。它可改变数百种底物的精氨酸甲基化状态,从不对称二甲基精氨酸(ADMA)转变为单甲基精氨酸(MMA)和对称二甲基精氨酸(SDMA)[1] - 作为单药疗法,GSK3368715在体外对多种血液和实体肿瘤细胞系具有抗增殖作用。在细胞Western实验中,将RKO细胞接种于透明底384孔板,用GSK3368715的20点两倍稀释系列(29,325.5至0.03 nM)或0.15% DMSO处理,随后在37°C、5% CO₂条件下孵育3天,结果显示其具有较强的抗增殖活性[1] - GSK3368715与PRMT5抑制剂(如GSK3326595)协同作用,增强对MTAP缺陷型癌细胞系的抗增殖效果,与单药处理相比,细胞活力显著降低[1] |
| 体内研究 (In Vivo) |
在所有测试剂量下,GSK3368715 (EPZ019997) 均显着抑制 BxPC3 异种移植物的生长,150 mg/kg 和 300 mg/kg 剂量组的肿瘤生长分别减少 78% 和 97% [1]。
- GSK3368715在体内肿瘤模型中可完全抑制肿瘤生长或诱导肿瘤消退。在MTAP缺陷型异种移植模型中,给予GSK3368715可显著抑制肿瘤生长,且对MTAP缺陷型肿瘤的疗效优于MTAP正常型肿瘤[1] - GSK3368715与PRMT5抑制剂(GSK3326595)联合使用时,在MTAP缺陷型异种移植物中显示出增强的抗肿瘤活性,与单药相比,肿瘤消退更明显[1] |
| 酶活实验 |
高通量筛选[1]
I型PRMT抑制剂是通过筛选Epizyme的专有HMT偏向文库发现的(Mitchell等人,2015)。总之,将化合物与PRMT1在室温下孵育30分钟(384孔板),并在添加SAM和肽后引发反应。最终测定条件为0.75 nM PRMT1(NP_001527.3,GST-PRMT1氨基酸1-371)、200 nM 3H-SAM(比活性80 Ci/mmol)、1.5μM SAM和20 nM肽(Biotin-Ahx-RLARRGGVKRISGLI-NH2,21st Century Biochemicals)在20 mM bincine(pH 7.6)、1 M TCEP、0.005%牛皮明胶、0.002%Tween-20和2%DMSO中的溶液。通过添加SAM(最终400μM)来猝灭反应。将终止的反应转移到Streptavidin包被的Flashplate中,孵育至少1小时,然后使用Biotek ELx405洗板器用0.1%Tween-20洗涤板。使用PerkinElmer TopCount平板读取器测量结合到闪板上的3H肽的量。 PRMT生化分析[1] 所有测定均在96孔板中用化合物或DMSO预缓冲(49x,2%最终)进行。PRMT1、PRMT3、PRMT6和PRMT8的测定使用H4 1-21肽和由50mM Tris(pH 8)、0.002%Tween-20、0.5mM EDTA和1mM DTT组成的缓冲液。简言之,Flag-his-tev-PRMT8(61-394)在杆状病毒表达系统中表达,并使用Ni-NTA琼脂糖亲和层析和Superdex 200凝胶过滤层析纯化。对于所有测定,列出的最终腺苷-L-蛋氨酸(SAM)浓度包含未标记的SAM和3H-SAM的混合物。所有反应在加入SAH(最终0.5mM)后终止。[1] 对于竞争研究,将底物加入到复合板中,然后加入酶。对于SAM竞争研究,最终测定浓度由2 nM PRMT1、40 nM肽和滴定SAM(50-8000 nM)组成。对于肽竞争研究,最终测定浓度由2 nM PRMT1、1000 nM和滴定肽(1.6-1000 nM)组成。在骤冷之前,将反应物在室温下孵育18分钟。[1] 对于时间依赖性研究,将酶/SAM混合物添加到化合物板中,并在添加肽之前孵育3-60分钟。对于无预培养测定,将肽加入到化合物板中,然后加入酶/SAM混合物以引发反应。最终PRMT1测定浓度为0.5 nM PRMT1、40 nM肽和1100 nM SAM。反应在室温下孵育20分钟,然后淬灭。 甲基转移酶生化测定法[1] 总之,将甲基转移酶加入底物溶液中并轻轻混合。底物根据测试的甲基转移酶而变化,并且是核小体、核心组蛋白、组蛋白H3、组蛋白H4或H3 1-21肽。加入化合物(最终10μM)并在室温下孵育10分钟。加入3H-SAM(1μM)后开始反应,并在30°C下孵育1小时。将反应混合物输送到P81滤纸中,并用PBS洗涤以通过HotSpot专有技术进行检测。 |
| 细胞实验 |
细胞内蛋白质[1]
将RKO细胞接种在透明底部384孔板中,并用GSK3368715(29325.5至0.03nM)或0.15%DMSO的20点两倍稀释系列处理。将平板在37°C、5%CO2中孵育3天。细胞在室温下用冰冷的甲醇固定30分钟,用磷酸盐缓冲盐水(PBS)洗涤,然后在室温下与奥德赛阻断缓冲液孵育1小时。去除封闭缓冲液,将细胞与在封闭缓冲液加0.1%吐温-20中稀释的兔抗单甲基精氨酸和小鼠抗α-微管蛋白在4°C下孵育过夜。PBS洗涤后,施加第二抗体IRDye 800CW山羊抗兔IgG(H+L)和IRDye 680RD山羊抗小鼠IgG(H+R)1小时。用PBS,然后用ddH2O彻底洗涤板,并使其在室温下干燥。使用Li-Cor Odyssey成像仪和软件对板进行扫描和分析。通过使用Microsoft Excel将MMA的积分强度除以微管蛋白的积分强度来确定相对MMA表达。然后根据化合物的对数浓度绘制MMA水平,并使用GraphPad Prism 6.0使用4参数拟合方程绘制。 细胞增殖测定[1] 如前所述评估对GSK3368712和GSK3368715的生长抑制反应(McCabe等人,2012)。用四参数方程拟合数据以生成浓度响应曲线。生长IC50(gIC50)和生长IC100(gIC100)分别是实现50%和100%生长抑制的点。生长抑制是最大抑制百分比,计算为100-((ymin-100)/(ymax-100)*100)。Ymin-T0值是通过从曲线上的Ymin值减去T0值(100%)来计算的,并且是净群体细胞生长或死亡的度量。生长死亡指数(GDI)是Ymin-T0和前体最大抑制的复合表示。如果Ymin-T0值为负,则GDI等于Ymin-TO;否则,GDI表示相对于DMSO对照(ymax)和(ymin):(ymin-100)/(ymax-100)*100)的剩余细胞分数。每个测定至少评估两个生物重复。 对细胞增殖协同效应的评估[1] 如上所述,进行GSK3368715(或GSK3368712)和GSK3203591的双重滴定6天,不同之处在于给细胞施用浓度为0.3至10000nM的两种试剂的16pt、2倍稀释基质。单剂滴定在帕拉列尔中进行。使用每种组合的生长抑制值和如前所述确定的协同作用得分进行布利斯独立性分析(McGrath等人,2016)<小时> 细胞周期分析[1] Toledo或OCI-Ly1-DLBCL细胞系用5点、10倍稀释系列GSK3368715或0.1%DMSO处理10天。在第3天、第5天、第7天和第10天,分离细胞核,并根据制造商的说明使用CycleTEST PLUS DNA试剂盒(Becton Dickinson)用碘化丙啶对DNA进行染色。使用Becton Dickinson FACS Calibur流式细胞仪测量荧光。使用FlowJo软件通过Watson Pragmatic数学模型确定细胞周期相分布。 Caspase 3/7测定[1] GSK3368715处理对细胞胱天蛋白酶3/7活性的影响用胱天蛋白酶-Glo™3/7测定试剂盒测定。根据制造商的说明进行分析。如细胞增殖测定所述,将细胞接种并给予GSK3368715或DMSO。在每个时间点,将CellTiter-Glo试剂添加到重复板中以评估细胞活力,将Caspase-Glo 3/7试剂添加到另一对重复板中来评估细胞死亡。用EnVision平板阅读器测量发光信号。GSK3368715和DSMO的Caspase 3/7Glo和CTG值被背景减去。为了说明细胞数量,然后将每个剂量的Caspase 3/7Glo值标准化为其相应的CTG值。对于每一剂量的GSK3368715,归一化的Caspase 3/7Glo值表示为DMSO Caspase 3/8 Glo平均值的倍数增加。然后对每个生物复制品的复制板的折叠增加进行平均。 - 抗增殖实验:将RKO细胞接种于透明底384孔板,用系列浓度的GSK3368715或0.15% DMSO(对照)处理。在37°C、5% CO₂条件下孵育3天后,用冰甲醇在室温下固定细胞30分钟,PBS洗涤,并用封闭缓冲液室温孵育1小时,通过适当检测方法评估细胞活力和增殖[1] - 协同实验:将MTAP缺陷型和MTAP正常型癌细胞分别用GSK3368715单药、PRMT5抑制剂单药或两者联合处理。72小时后检测细胞活力,计算联合指数(CI)以确定协同作用[1] |
| 动物实验 |
In vivo antitumor efficacy study[1]
Efficacy studies of GSK3368712 in a pancreatic patient derived xenograft model (PAXF 2076) were carried out at Charles River Discovery Research Services Germany. Tumor fragments were implanted into Female NMRI nu/nu mice (NMRI-Foxn1nu). Animals and tumor implants were monitored daily until solid tumor growth was detectable in a sufficient number of animals. Following randomization, animals were assigned into study groups and dosed once daily with vehicle or GSK3368712. Toxicology Assessment[1] The toxicological profile of once-daily, oral dosing of GSK3368715 was evaluated in rising and repeat dose toxicity studies. Doses up to the maximal tolerated dose were evaluated in dose range studies. Studies were conducted using pharmacologically relevant rodent (rat; 10-12 week old Wistar:Han; n=10-16 per sex per group) and non-rodent (dog; 10-12 month old beagle; n=3-5 per sex per group) species. Assessments were GLP compliant and consistent with ICH S9 guidance. All studies were conducted in accordance with the GSK Policy on the Care, Welfare and Treatment of Laboratory Animals and were reviewed by the Institutional Animal Care and Use Committee at GSK. Pharmacokinetic study[1] Pharmacokinetic analysis of GSK3368715 and GSK3368712 revealed that both compounds had suitable PK properties for oral administration and in vivo assessment of anti-tumor activity (Table S3). In toxicology studies conducted in rats and dogs, primary on-target toxicity affected the gastrointestinal tract and mild-to-moderate changes to hematopoetic lineages (Table S4), while doses used in mice were well tolerated. The efficacy of type I PRMTi in mice bearing xenografts of cell lines that had cytotoxic responses was examined. The Toledo DLBCL cell line has a cytotoxic response to GSK3368715 with a gIC50 of 59 nM in vitro (Figure 2C). Once-daily administration of GSK3368715 induced dose-dependent inhibition of Toledo tumor growth, with tumor regression in mice treated with >75 mg/kg (Figure 2D). The BxPC3 pancreatic adenocarcinoma cell line has a gIC50 of 2,100 nM, and was cytotoxic at concentrations above 10 μM GSK3368715 (Figure 2E). Once-daily administration of type I PRMTi had significant effects on the growth of BxPC3 xenografts at all doses tested, reducing tumor growth by 78% and 97% in the 150- and 300-mg/kg dose groups, respectively (Figure 2F). Efficacy studies with once-daily administration of 150 mg/kg GSK3368715 in cell line xenograft models of clear cell renal carcinoma (ACHN) and triple-negative breast cancer (MDA-MB-468) revealed tumor growth inhibition of 98% and 85%, respectively (Figures S2F and S2G). In a patient-derived xenograft model of pancreatic adenocarcinoma, type I PRMTi had significant effects on tumor growth, with inhibition >90% in a subset of animals within the 300-mg/kg cohort (Figure 2G).These data demonstrate that GSK3368715 has potent, anti-proliferative activity across cell lines representing a range of solid and hematological malignancies and can completely inhibit tumor growth or cause regressions of tumor models in vivo. Xenograft models: Nude mice bearing MTAP-deficient or MTAP-proficient tumor xenografts were administered GSK3368715 via oral gavage at specified doses. For combination studies, mice received GSK3368715 and PRMT5 inhibitor (GSK3326595) according to a predefined schedule. Tumor volume was measured regularly, and mice were monitored for survival and body weight changes [1] |
| 参考文献 | |
| 其他信息 |
Type I protein arginine methyltransferases (PRMTs) catalyze asymmetric dimethylation of arginines on proteins. Type I PRMTs and their substrates have been implicated in human cancers, suggesting inhibition of type I PRMTs may offer a therapeutic approach for oncology. The current report describes GSK3368715 (EPZ019997), a potent, reversible type I PRMT inhibitor with anti-tumor effects in human cancer models. Inhibition of PRMT5, the predominant type II PRMT, produces synergistic cancer cell growth inhibition when combined with GSK3368715. Interestingly, deletion of the methylthioadenosine phosphorylase gene (MTAP) results in accumulation of the metabolite 2-methylthioadenosine, an endogenous inhibitor of PRMT5, and correlates with sensitivity to GSK3368715 in cell lines. These data provide rationale to explore MTAP status as a biomarker strategy for patient selection.[1]
• GSK3368715 is a potent inhibitor of type I protein arginine methyltransferases • GSK3368715 alters exon usage and has activity against multiple cancer models • GSK3368715 synergizes with the PRMT5 inhibitor GSK3326595 to inhibit tumor growth • MTAP gene deficiency impairs PRMT5 activity, sensitizing cancer cells to GSK3368715 - Type I PRMTs catalyze asymmetric dimethylation of arginine residues, and their dysregulation is associated with human cancers. GSK3368715 targets these enzymes to disrupt oncogenic signaling [1] - MTAP deficiency leads to accumulation of 2-methylthioadenosine (MTA), an endogenous PRMT5 inhibitor, rendering MTAP-deficient cancers more sensitive to GSK3368715 and enhancing its synergism with PRMT5 inhibitors [1] |
| 分子式 |
C20H41CL3N4O2
|
|---|---|
| 分子量 |
475.9241
|
| 精确质量 |
366.3
|
| 元素分析 |
C, 65.54; H, 10.45; N, 15.29; O, 8.73
|
| CAS号 |
1629013-22-4
|
| 相关CAS号 |
GSK3368715 dihydrochloride;1628925-77-8;GSK3368715 trihydrochloride;2227587-26-8; 1629013-22-4; 2227587-25-7 (HCl); 2227587-26-8 (3HCl)
|
| PubChem CID |
90462880
|
| 外观&性状 |
Solid powder
|
| LogP |
1.5
|
| tPSA |
62.4Ų
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
12
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
365
|
| 定义原子立体中心数目 |
0
|
| SMILES |
Cl[H].Cl[H].Cl[H].O(C([H])([H])C([H])([H])[H])C([H])([H])C1(C([H])([H])OC([H])([H])C([H])([H])[H])C([H])([H])C([H])([H])C([H])(C2=C(C([H])=NN2[H])C([H])([H])N(C([H])([H])[H])C([H])([H])C([H])([H])N([H])C([H])([H])[H])C([H])([H])C1([H])[H]
|
| InChi Key |
SPEGERVLTUWZPA-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C20H38N4O2/c1-5-25-15-20(16-26-6-2)9-7-17(8-10-20)19-18(13-22-23-19)14-24(4)12-11-21-3/h13,17,21H,5-12,14-16H2,1-4H3,(H,22,23)
|
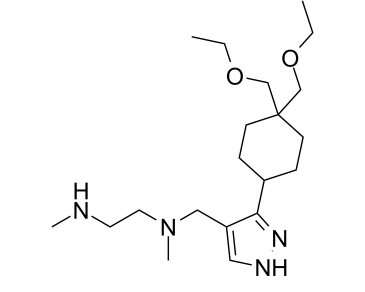
| 化学名 |
N1-((3-(4,4-bis(ethoxymethyl)cyclohexyl)-1H-pyrazol-4-yl)methyl)-N1,N2-dimethylethane-1,2-diamine
|
| 别名 |
EPZ019997;SK3368715; GSK-3368715; GSK 3368715; EPZ019997; EPZ-019997; GSK 3368715; EPZ019997; EPZ-019997;GSK-3368715; EPZ 019997
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1012 mL | 10.5060 mL | 21.0119 mL | |
| 5 mM | 0.4202 mL | 2.1012 mL | 4.2024 mL | |
| 10 mM | 0.2101 mL | 1.0506 mL | 2.1012 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。