| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 靶点 |
Integrin CD11b/CD18
Leukadherin-1 (LA1) acts on integrin CD11b/CD18 (Mac-1/CR3) as an agonist [1] Leukadherin-1 (LA1) targets integrin CD11b/CD18 (Mac-1) as a small-molecule agonist [2] Leukadherin-1 (LA1) is a specific agonist of complement receptor 3 (CR3, CD11b/CD18) [3] |
|---|---|
| 体外研究 (In Vitro) |
白细胞表面整合素 CD11b/CD18(αMβ2;CR3;Mac-1)的一种特殊激动剂是 leukadherin-1。当用 leukadherin-1 预处理时,单核因子刺激的自然杀伤 (NK) 细胞分泌较少的干扰素 (IFN)-γ、肿瘤坏死因子 (TNF) 和巨噬细胞炎症蛋白 (MIP)-1β。用 leukadherin-1 预处理还可减少 TLR-2 和 TLR-7/8 激活的单核细胞释放 TNF、IL-1β 和 IL-6 [3]。
白细胞粘附素-1是一种特殊的小分子CR3激动剂,在血管损伤和炎症的动物模型中显示出治疗前景。我们发现,白细胞粘附素-1预处理可减少单因子刺激的NK细胞分泌干扰素(IFN)-γ、肿瘤坏死因子(TNF)和巨噬细胞炎性蛋白(MIP)-1β。它与白细胞介素(IL)-12+IL-15刺激后磷酸化信号转导子和转录激活子(pSTAT)-5的减少有关(P<0.02),与IL-12+IL-18刺激后IL-10分泌的增加有关(P<0.001)。白细胞粘附素-1预处理还降低了Toll样受体(TLR)-2和TLR-7/8刺激的单核细胞分泌IL-1β、IL-6和TNF的能力(均P<0.01)。R77H变体不影响NK细胞对使用纯合供体离体细胞的白细胞粘附素-1的反应;与最近的一份报告相反,该变体也没有影响这些细胞类型的CR3表达。这些数据扩展了我们对CR3生物学的理解,表明激活能有效地改变先天免疫炎症信号传导,包括以前未记录的NK细胞功能中的作用。我们讨论了这与SLE发病机制的潜在相关性。白细胞粘附素-1似乎介导其抗炎作用,而与CR3的SLE风险基因型无关,这为其评估白细胞粘附蛋白-1作为自身免疫性疾病的潜在治疗方法提供了进一步的证据[3]。 Leukadherin-1 (LA1)可剂量依赖性增强转染CD11b/CD18的K562细胞与固定化纤维蛋白原的黏附;同时提升原代人/小鼠中性粒细胞与纤维蛋白原的黏附能力(该效应可被CD11b/CD18阻断抗体IB4、44a消除,且在CD11b基因敲除(CD11b⁻/⁻)中性粒细胞中未观察到)[2] - Leukadherin-1 (LA1)可降低fMLP刺激的原代小鼠中性粒细胞、MCP-1刺激的THP-1细胞(跨TNF-α激活的HUVEC单层)的趋化能力(平均位移、速度、定向持续性均降低)及跨内皮迁移能力;它可稳定迁移中性粒细胞尾部的CD11b定位,减少其侧向及跨内皮移动[2] - Leukadherin-1 (LA1)预处理可减少IL-12+IL-15或IL-12+IL-18刺激的人NK细胞分泌IFN-γ、TNF、MIP-1β;降低IL-12+IL-15刺激的NK细胞中磷酸化STAT5(pSTAT5)水平,增加IL-12+IL-18刺激的NK细胞分泌IL-10(对pSTAT4水平无影响)[3] - Leukadherin-1 (LA1)预处理可减少TLR-2激动剂(Pam3csk4)或TLR-7/8激动剂(R848)刺激的人单核细胞分泌IL-1β、IL-6、TNF;增加R848刺激的单核细胞分泌IL-12,增加Pam3csk4刺激的单核细胞分泌IL-10和IL-12[3] |
| 体内研究 (In Vivo) |
在支气管肺发育不良 (BPD) 实验模型中,leukadherin-1(1 mg/kg;腹腔注射;每天两次,持续 14 天)有助于预防高氧诱导的新生儿肺损伤[1]。
给予白细胞粘附素-1(LA1)有利于预防高氧诱导的新生儿肺损伤,这是BPD的实验模型。新生大鼠暴露于常氧(21%O2)或高氧(85%O2)环境中,每天两次腹腔注射LA1或安慰剂,持续14天。在安慰剂存在的情况下,高氧暴露导致中性粒细胞和巨噬细胞流入肺泡气室的数量急剧增加。白细胞内流的增加伴随着肺泡化和血管生成的减少,以及肺血管重塑和肺动脉高压(PH)的增加,这是BPD的病理特征。然而,LA1的给药减少了高氧期间肺部巨噬细胞的浸润。此外,LA1治疗改善了肺泡化和血管生成,减少了肺血管重塑和肺动脉高压。这些数据表明,白细胞募集在高氧诱导的BPD实验模型中起着重要作用。使用整合素激动剂LA1靶向白细胞运输有利于预防肺部炎症,并在高氧期间保护肺泡和血管结构。因此,靶向整合素介导的白细胞募集和炎症可能为预防和治疗早产儿BPD提供一种新策略。[1] 在这里,研究人员使用了一种替代策略,通过用小分子激动剂激活CD11b/CD18来抑制白细胞募集,我们称之为白细胞粘附素(如白细胞粘附蛋白-1(LA1))。这些化合物增加了转染细胞和原代人和小鼠中性粒细胞CD11b/CD18依赖性细胞粘附的程度,导致趋化性和跨内皮迁移减少。白细胞粘附素还减少了大鼠损伤后的白细胞募集和动脉狭窄。此外,与已知的整合素拮抗剂相比,白细胞粘附素在实验性肾炎的小鼠模型中更好地保护了肾功能。白细胞粘附素通过增加白细胞与炎症内皮的粘附来抑制白细胞募集,而阻断抗体可以逆转这一过程。因此,我们提出CD11b/CD18的药理学激活为炎症性疾病提供了一种替代治疗方法。[2] 与用赋形剂预处理的小鼠相比,在注射巯基乙酸前30分钟服用LA1可显著减少40%的中性粒细胞积聚量(P<0.05),而LA2可减少65%(P<0.0001),LA3可减少55%[2]。 新生大鼠暴露于85%氧浓度(高氧)并接受Leukadherin-1 (LA1)(每日两次腹腔注射,持续14天)处理后,肺部巨噬细胞浸润减少(BAL液及肺组织切片中Mac3⁺细胞数降低),肺匀浆中MCP-1水平下降,肺泡发育改善(放射状肺泡计数增加、平均线性截距降低),血管生成增强(血管密度及VEGF表达升高),肺血管重构减轻(外周血管肌化程度、中膜厚度、WISP-1表达降低),肺动脉高压缓解(右心室肥厚减轻;右心室收缩压(RVSP)呈非显著性下降)[1] - Leukadherin-1 (LA1)可减少硫代乙醇酸盐诱导的小鼠腹膜炎模型中(4小时)腹腔中性粒细胞募集(在CD11b⁻/⁻小鼠中无此效应);降低大鼠颈动脉球囊损伤后21天动脉内膜增厚程度(内膜/中膜比降低),减少损伤后3天动脉中CD68⁺巨噬细胞浸润[2] - 在小鼠实验性肾炎模型中,Leukadherin-1 (LA1)相较于CD11b/CD18拮抗剂M1/70,能更好地保护肾功能(降低蛋白尿及肾小球中性粒细胞浸润)[2] - Leukadherin-1 (LA1)可减少斑马鱼尾鳍损伤模型中损伤部位的中性粒细胞聚集;小鼠提睾肌静脉活体显微镜显示,LA1可增加白细胞黏附、降低滚动速度、减少跨内皮迁移效率(TEM)[2] |
| 酶活实验 |
Leukadherin-1,也称为 LA1,是补体受体 3 (CR3) 和白细胞表面整合素 CD11b/CD18 的新型特异性激动剂,可增强白细胞对配体和血管内皮的粘附,从而减少白细胞跨内皮迁移和流入损伤网站。补体受体 3(CR3、CD11b/CD18)是一种多功能受体,主要表达于骨髓细胞和自然杀伤 (NK) 细胞。 Leukadherin-1 (LA1) 不调节信号转导子和转录激活子 (STAT)-4 磷酸化。 Leukadherin-1 调节 TLR-2 和 TLR-7/8 诱导的单核细胞细胞因子分泌。使用整合素激动剂 LA1 靶向白细胞运输,有助于预防肺部炎症并在高氧状态下保护肺泡和血管结构。因此,针对整合素介导的白细胞募集和炎症可能为预防和治疗早产儿 BPD 提供新策略。
αA结构域配体结合分析[2] MaxiSorp 96孔板在10 mM磷酸盐缓冲盐水(PBS,pH 7.4)中用纤维蛋白原(每孔1μg)涂覆过夜,并用PBS中的1%BSA封闭。纯化的GST标记的αA结构域(5μg/ml溶液的每孔50μl)与固定的纤维蛋白原的结合在基于TBS的测定缓冲液(TBS含有0.1%BSA、1 mM MgCl2、1 mM CaCl2和0.05%吐温20)(TBS Ca/Mg缓冲液)中在室温下进行1小时。αA结构域也被添加到板上的未涂覆孔中,以估计每个孔中可以捕获和检测的最大蛋白质量,从而进行数据归一化。通过用TBS Ca/Mg缓冲液洗涤孔两次来去除未结合的αA结构域。随后,通过与辣根过氧化物酶偶联的抗GST抗体(GE,1:2000稀释)孵育1小时来测定结合蛋白的量。通过用TBS Ca/Mg缓冲液洗涤孔两次来去除未结合的抗体。根据制造商的方案,用3,3′,5,5′-四甲基联苯胺(TMB)底物试剂盒检测结合蛋白。用SpectraMax M5分光光度计读取吸光度)。吸光度值被归一化,使得输入αA结构域孔的平均吸光度设置为100%,结果以野生型αA结构区总输入量的百分比表示。在三个重复的井中进行了分析,显示的数据来自至少三个独立实验中的一个。 CD11b/CD18介导的细胞黏附实验:将转染CD11b/CD18的K562细胞或原代中性粒细胞与系列浓度的Leukadherin-1 (LA1)(或赋形剂DMSO)在Ca²⁺/Mg²⁺存在下孵育(以Mn²⁺为阳性对照),随后加入包被纤维蛋白原的培养板;洗去未黏附细胞后定量黏附细胞数;采用CD11b/CD18阻断抗体(IB4/44a)或CD11b⁻/⁻中性粒细胞作为对照,验证CD11b/CD18的特异性[2] |
| 细胞实验 |
刺激并培养 18 小时(单核细胞)或 24 小时(NK 细胞)后对上清液细胞因子进行定量。除了基于珠子的刺激外,所有实验均在 96 孔板格式中使用 100 µL 细胞进行。 NK 细胞刺激物添加如下:(1) Syk 抑制剂 (1 μM),(2) Leukadherin-1 或二甲基亚砜 (DMSO)(载体对照)(7.5 μM)。显示可诱导约 82% 的最大反应,且脱靶效应可忽略不计,(3) 抗 CD210 或同种型对照 (5 µg/mL),(4) 用以下组合刺激 Leukadherin-1 NK 细胞后 30-45 分钟IL-12 (10 ng/mL)、IL-15 (30 ng/mL) 或 IL-18 (10 ng/mL):IL-12 + IL-15 或 IL-12 + IL-18。使用 pam3csk4(TLR-2 激动剂,300 ng/mL)或 R848(TLR-7/8 激动剂,2 µg/mL)刺激单核细胞。上清液在-80°C 下保存。
用补体iC3b包被的绵羊红细胞(EiC3bs)进行吞噬试验[2] 如前所述,制备了涂有补体iC3b的绵羊红细胞,并将其用于吞噬试验。将包被的红细胞(EiC3bs)稀释至1.5×107至6×107个细胞/ml的浓度。将K562 CD11b/CD18细胞在TBS中洗涤两次,并重新悬浮至1×106/ml,其中40μl(4×104个细胞)与总体积为100μl的EiC3bs(1.2×106)在37°C下悬浮培养25分钟,CaCl2和MgCl2各有1 mM(在没有或存在50至100μM白细胞粘附素-1(LA1)、LA2或LA3的情况下),在1 mM MnCl2或10 mM EDTA中。如前所述,通过相差显微镜目视分析玫瑰花结的形成[多个红细胞(EiC3bs)与单个K562细胞的结合]来检测结合。对于评分,只有与≥3个EiC3bs结合的K562细胞被评为阳性,在每种情况下,在多个领域检查了>200个细胞。结合结果显示了田间所有显示玫瑰花结的细胞的百分比,以直方图的形式报告,表示三次实验的平均值±SEM;所示数据来自至少三个独立实验中的一个。 细胞活力测定[2] 使用市售试剂和试剂盒进行细胞活力测定。简而言之,将1×104 K562 CD11b/CD18细胞或野生型B6中性粒细胞在96孔板的每个孔中与越来越多的指定化合物一起孵育,根据制造商的说明,在孵育4小时(中性粒细胞)或24小时(K562细胞)后,用MTS试剂测定活细胞的数量。使用SpectraMaxM5分光光度计读取测定板。数据代表至少两个独立的实验。 蛋白质印迹分析[2] 将K562 CD11b/CD18细胞与白细胞粘附素-1(LA1)、LA2或LA3(15μM)或纤维蛋白原(200μg)在37°C的无血清培养基中孵育1小时。细胞裂解物在10%SDS-PAGE凝胶上分离,并通过既定的方案转移到聚偏二氟乙烯膜上。将膜与1:1000稀释的抗磷酸化细胞外信号调节激酶1/2(ERK1/2)抗体(Thr202/Tyr204)一起孵育,用Reblot温和剥离溶液剥离,然后孵育,首先用抗总ERK1/2抗体,然后用抗甘油醛-3-磷酸脱氢酶(GAPDH)抗体,并根据制造商的说明进行开发。所提供的数据代表了至少三个独立的实验。 中性粒细胞趋化实验:原代野生型小鼠中性粒细胞经Leukadherin-1 (LA1)(或DMSO)处理后,置于含fMLP梯度的Zigmond小室中;通过延时视频显微镜追踪细胞迁移,量化迁移参数(平均位移、速度、定向持续性);采用免疫荧光(CD11b/F-actin染色)分析CD11b定位[2] - 跨内皮迁移实验:THP-1细胞经Leukadherin-1 (LA1)(或DMSO)预处理后,加入含TNF-α激活的HUVEC单层的transwell小室(下室含MCP-1梯度);通过共聚焦显微镜计数黏附及迁移的THP-1细胞数[2] - NK细胞细胞因子分泌实验:原代人NK细胞经Leukadherin-1 (LA1)(或DMSO)预处理30分钟后,用IL-12+IL-15或IL-12+IL-18刺激24小时;采用流式细胞微球阵列技术(CBA)检测上清中细胞因子(IFN-γ、TNF、MIP-1β、IL-10)水平;通过磷酸流式细胞术(Phosflow)在多个时间点(10分钟至2小时)检测pSTAT4/pSTAT5水平[3] - 单核细胞细胞因子分泌实验:原代人单核细胞经Leukadherin-1 (LA1)(或DMSO)预处理30分钟后,用TLR-2激动剂(Pam3csk4)或TLR-7/8激动剂(R848)刺激18小时;采用CBA定量上清中细胞因子(IL-1β、IL-6、TNF、IL-10、IL-12)水平[3] |
| 动物实验 |
Animal/Disease Models: Newborn Sprague Dawley rat pups[1]
Doses: 1 mg/kg Route of Administration: Ip; twice (two times) daily for 14 days Experimental Results: Beneficial on preventing the lung inflammatory response, improved alveolarization and vascular development, and decreased pulmonary vascular remodeling and PH in a hyperoxia-induced experimental model of BPD.\n \nAnimal Model and Experimental Protocol [1] \nNewborn Sprague Dawley rat pups (n = 12 per group) were randomized on Postnatal Day 2 into four groups: normoxia (21% O2) plus placebo, normoxia plus Leukadherin-1 (LA1), hyperoxia (85% O2) plus placebo, and hyperoxia plus Leukadherin-1 (LA1). Rat pups received Leukadherin-1 (LA1) (1 mg/kg, mixed in 2% DMSO) or placebo (2% DMSO) (equal volume) twice daily by intraperitoneal injection for 14 days. Pups were killed on Postnatal Day 15 for analyses.\n \nIn vivo peritonitis model [2] \nThioglycolate-induced peritonitis in 8- to 10-week-old wild-type B6 and CD11b−/− B6 mice was performed as previously described. Leukadherins were administered 30 min before intraperitoneal injection with 3% thioglycolate. Leukadherin-1 (LA1) and LA2 (200 μl of a 50 μM solution in saline) were administered intravenously, whereas LA3 was administered intraperitoneally (500 μl of a 50 μM solution in saline). To evaluate peritoneal neutrophil recruitment, we euthanized mice at 4, 12, or 24 hours after thioglycolate injection; the peritoneal lavage was collected, and the number of emigrated neutrophils was quantified by flow cytometric analysis for cells expressing both GR-1 and Mac-1 on the surface, as described previously \n \nAnti-GBM nephritis [2] \nExperimental anti-GBM nephritis was induced in wild-type B6 mice (n = 4 mice per group) by intraperitoneal injection of sheep antibody against rabbit GBM (0.5 ml per 20 g body weight per day for 2 consecutive days) as previously described. One group of animals was treated with leukadherins by daily intraperitoneal injection of Leukadherin-1 (LA1) (500 μl of a 50 μM solution in saline) starting at 2 hours before induction of nephritis and continuing until the end of the experiment. A group of animals was treated with a known antagonist, the blocking antibody M1/70, as described previously (76). Briefly, M1/70 (100 μg per injection) in a saline solution was injected intraperitoneally every other day starting at 2 hours before induction of nephritis and continuing until the end of the experiment. Urine collections were performed every 24 hours and analyzed for evidence of proteinuria on SDS-PAGE gels with BSA standards, as described previously. The amounts of creatinine were determined with a creatinine assay kit according to the manufacturer’s instructions. Mice were killed on days 0, 3, and 7, and renal biopsies were obtained from each animal. Tissue sections were fixed with 4% paraformaldehyde and were used for histochemical analyses and leukocyte enumeration.\n \nEvaluation of neutrophil sequestration [2] \nTo evaluate whether leukadherins induced tissue sequestration of neutrophils, we fixed various organs from wild-type B6 mice (n = 3 mice per group) with formalin and stained them with hematoxylin and eosin (H&E). The numbers of neutrophils in untreated and Leukadherin-1 (LA1)-treated animals were quantified from four random fields for each specimen at either 40× or 1000× magnification in a blinded fashion. Bone marrow was isolated as described previously. Briefly, mice were euthanized, and the femurs and tibia from both hind legs were removed and freed of soft tissue. The extreme ends of the bones were cut off, and a solution of RPMI 1640 containing 10% FBS was forced through the bone with a 27-gauge syringe needle. Cell clumps were dispersed, passed through an 80-μm filter, and collected by centrifugation. Red blood cells (RBCs) were removed by hypotonic lysis buffer followed by two washes with HBSS buffer containing 0.1% BSA. Neutrophil numbers were determined by flow cytometry as described earlier.\n \nIntravital microscopy [2] \nMice were given an intrascrotal injection of TNF-α (500 ng) in 0.25 ml of saline 3 hours before cremaster muscle exteriorization. Some animals also received intravenous injections of the blocking mAb M1/70 (30 μg per mouse) in 0.1 ml of saline and intraperitoneal injections of Leukadherin-1 (LA1) (100 μM) or DMSO in 0.5 ml of saline 30 min before injection with TNF-α. Mice were anesthetized with an intraperitoneal injection of ketamine (125 mg/kg), xylazine (12.5 mg/kg), and atropine sulfate (0.025 mg/kg) and placed on a 38°C heating pad. After tracheal intubation and cannulation of one carotid artery, the cremaster was exteriorized, pinned to the stage, and superfused with thermocontrolled bicarbonate-buffered saline (131.9 mM NaCl, 18 mM NaHCO3, 4.7 mM KCl, 2.0 mM CaCl2, and 1.2 mM MgCl2) equilibrated with 5% CO2 in N2. Cremaster muscles were illuminated with stroboscopic flash epi-illumination (DPS-1, Rapp OptoElectronic) and halogen transillumination. Microscopic observations were made on postcapillary venules with a diameter of between 20 and 40 mm by means of an intravital microscope with a saline immersion objective (SW 40/0.75). A charge-coupled device (CCD) camera (model SIT66, DAGE-MTI) was used for recording. In a limited analysis, cells adjacent to the venules were counted to determine the number of transmigrated neutrophils. The surface area (S) was calculated for each vessel as S = πdIν, where d is the diameter of the vessel and Iν is the length of the vessel. Adherent leukocytes were defined as those cells that were stationary for more than 30 s. Hyperoxia-induced neonatal lung injury model (rats): Newborn Sprague-Dawley rats were randomly assigned to normoxia (21% O₂) or hyperoxia (85% O₂) groups; hyperoxia-exposed rats received twice-daily intraperitoneal injections of Leukadherin-1 (LA1) or placebo for 14 consecutive days; at day 14, rats were euthanized, bronchoalveolar lavage (BAL) was collected to count total cells/macrophages, lung homogenates were prepared to measure MCP-1 levels, lung tissue sections were stained for histomorphometric analysis (radial alveolar count, mean linear intercept) and immunofluorescence (vWF/α-SMA for vascular density), and Western blotting was performed to detect VEGF/WISP-1 expression; right ventricular hypertrophy (RVH) and right ventricular systolic pressure (RVSP) were measured to assess pulmonary hypertension [1] - Thioglycolate-induced peritoneal inflammation model (mice): Wild-type or CD11b⁻/⁻ mice received intraperitoneal injection of thioglycolate (or saline as control) after pretreatment with Leukadherin-1 (LA1), LA2, LA3, or vehicle; peritoneal fluid was collected at 4/12/24 h post-injection to count neutrophils; organ distribution of neutrophils was analyzed in LA1-treated vs. DMSO-treated mice [2] - Rat arterial balloon injury model: Rats received intraperitoneal injection of Leukadherin-1 (LA1) or DMSO after balloon injury of carotid arteries; arteries were harvested at day 3 (for CD68⁺ macrophage quantification) or day 21 (for morphometric analysis of neointima-to-media ratio) [2] - Mouse nephritis model: Mice were treated with Leukadherin-1 (LA1), CD11b/CD18 antagonist M1/70, or saline; glomerular neutrophil counts and proteinuria levels were measured at multiple time points [2] - Zebrafish tailfin injury model: Zebrafish larvae were treated with Leukadherin-1 (LA1) or DMSO; tailfin injury was induced, and neutrophil accumulation at the injury site was quantified by fluorescence microscopy at 4 h post-injury [2] - Intravital microscopy (mouse cremaster muscle): Mice were treated with Leukadherin-1 (LA1) or DMSO, then cremaster muscle venules were stimulated with TNF-α; leukocyte rolling velocity, adhesion, and transendothelial migration (TEM) were quantified via intravital microscopy; M1/70 (blocking antibody) was used to reverse LA1-induced adhesion [2] |
| 参考文献 |
|
| 其他信息 |
Lung inflammation plays a key role in the pathogenesis of bronchopulmonary dysplasia (BPD), a chronic lung disease of premature infants. The challenge in BPD management is the lack of effective and safe antiinflammatory agents. Leukadherin-1 (LA1) is a novel agonist of the leukocyte surface integrin CD11b/CD18 that enhances leukocyte adhesion to ligands and vascular endothelium and thus reduces leukocyte transendothelial migration and influx to the injury sites. Its functional significance in preventing hyperoxia-induced neonatal lung injury is unknown. We tested the hypothesis that administration of LA1 is beneficial in preventing hyperoxia-induced neonatal lung injury, an experimental model of BPD. Newborn rats were exposed to normoxia (21% O2) or hyperoxia (85% O2) and received twice-daily intraperitoneal injection of LA1 or placebo for 14 days. Hyperoxia exposure in the presence of the placebo resulted in a drastic increase in the influx of neutrophils and macrophages into the alveolar airspaces. This increased leukocyte influx was accompanied by decreased alveolarization and angiogenesis and increased pulmonary vascular remodeling and pulmonary hypertension (PH), the pathological hallmarks of BPD. However, administration of LA1 decreased macrophage infiltration in the lungs during hyperoxia. Furthermore, treatment with LA1 improved alveolarization and angiogenesis and decreased pulmonary vascular remodeling and PH. These data indicate that leukocyte recruitment plays an important role in the experimental model of BPD induced by hyperoxia. Targeting leukocyte trafficking using LA1, an integrin agonist, is beneficial in preventing lung inflammation and protecting alveolar and vascular structures during hyperoxia. Thus, targeting integrin-mediated leukocyte recruitment and inflammation may provide a novel strategy in preventing and treating BPD in preterm infants.[1]
The integrin CD11b/CD18 (also known as Mac-1), which is a heterodimer of the α(M) (CD11b) and β(2) (CD18) subunits, is critical for leukocyte adhesion and migration and for immune functions. Blocking integrin-mediated leukocyte adhesion, although beneficial in experimental models, has had limited success in treating inflammatory diseases in humans. Here, we used an alternative strategy of inhibiting leukocyte recruitment by activating CD11b/CD18 with small-molecule agonists, which we term leukadherins. These compounds increased the extent of CD11b/CD18-dependent cell adhesion of transfected cells and of primary human and mouse neutrophils, which resulted in decreased chemotaxis and transendothelial migration. Leukadherins also decreased leukocyte recruitment and reduced arterial narrowing after injury in rats. Moreover, compared to a known integrin antagonist, leukadherins better preserved kidney function in a mouse model of experimental nephritis. Leukadherins inhibited leukocyte recruitment by increasing leukocyte adhesion to the inflamed endothelium, which was reversed with a blocking antibody. Thus, we propose that pharmacological activation of CD11b/CD18 offers an alternative therapeutic approach for inflammatory diseases.[2] Our results demonstrate that CR3 is a negative regulator of this inflammatory loop and that Leukadherin‐1 is capable of marked NK cell cytokine down‐regulation. Furthermore Leukadherin‐1 is equally capable of down‐regulating signalling even in cells that are homozygous for the genetically encoded under‐functioning variant of CR3, presumably because the mechanism of CR3 signal transduction is different following engagement with this small molecule compound compared with natural ligand. We did not demonstrate any significant effect of Leukadherin‐1 in modulating cytokine production following engagement of CD16 on NK cells. Whether this would also apply to other CD16‐mediated functions, such as antibody‐dependent cellular cytotoxicity (ADCC), would require further investigation. In addition to documenting ex‐vivo effects of Leukadherin‐1 we have, in part, explained the mechanism in NK cells by demonstrating an inhibition of IL‐15‐induced phosphorylation of the transcription factor STAT‐5. Our data suggest that Leukadherin‐1 interferes with events that take place upstream of, or during, STAT‐5 phosphorylation – such as Janus kinase (JAK) phosphorylation or JAK/STAT association – rather than through a mechanism of STAT dephosphorylation. Activation of JAK‐1 and JAK‐3 associated with the IL‐2Rβ and γc‐chains, respectively, has been shown to be crucial for STAT‐5 tyrosine phosphorylation in response to IL‐15. A CR3‐mediated effect on JAKs has been shown recently in human monocytes/macrophages, in which CR3 activation with anti‐CD18 or anti‐CD11b antibodies blocks the phosphorylation of IL‐13 receptor‐associated JAK‐2 and tyrosine kinase‐2 (TYK‐2) kinases, which further inhibits the downstream tyrosine and serine phosphorylation of STAT‐1, STAT‐3 and STAT‐6. Further work is needed to determine the upstream mechanism through which the Leukadherin‐1‐mediated inhibition of STAT‐5 phosphorylation occurs in NK cells.[3] Leukadherin-1 (LA1) is a novel small-molecule agonist of leukocyte surface integrin CD11b/CD18 (Mac-1/CR3); it enhances leukocyte adhesion to vascular endothelium and ligands, thereby reducing leukocyte transendothelial migration and influx to injury sites [1] - Unlike integrin antagonists (which block leukocyte adhesion), Leukadherin-1 (LA1) reduces inflammatory disease by increasing leukocyte adhesion to inflamed endothelium (stabilizing adhesion, reducing migration), and it better preserves organ function than antagonists in experimental nephritis [2] - Leukadherin-1 (LA1) modulates innate immune signalling in humans: it suppresses pro-inflammatory cytokine secretion by NK cells and monocytes, with no differential effect based on the SLE-associated CD11b R77H polymorphism (the variant does not affect NK cell/monocyte response to LA1 or CD11b surface expression) [3] - Leukadherin-1 (LA1) is a potential therapeutic for inflammatory diseases (e.g., bronchopulmonary dysplasia in preterm infants, vascular injury, nephritis, autoimmune diseases like SLE) [1,2,3] |
| 分子式 |
C22H15NO4S2
|
|
|---|---|---|
| 分子量 |
421.49
|
|
| 精确质量 |
421.044
|
|
| 元素分析 |
C, 62.69; H, 3.59; N, 3.32; O, 15.18; S, 15.21
|
|
| CAS号 |
344897-95-6
|
|
| 相关CAS号 |
(Z)-Leukadherin-1;2055362-72-4;ADH-503;2055362-74-6
|
|
| PubChem CID |
5342077
|
|
| 外观&性状 |
Light yellow to yellow solid powder
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 沸点 |
634.7±65.0 °C at 760 mmHg
|
|
| 闪点 |
337.6±34.3 °C
|
|
| 蒸汽压 |
0.0±2.0 mmHg at 25°C
|
|
| 折射率 |
1.756
|
|
| LogP |
4.88
|
|
| tPSA |
128
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
29
|
|
| 分子复杂度/Complexity |
680
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
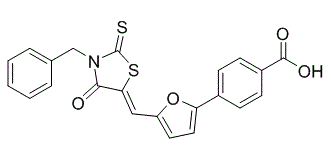
S1C(N(C(/C/1=C(\[H])/C1=C([H])C([H])=C(C2C([H])=C([H])C(C(=O)O[H])=C([H])C=2[H])O1)=O)C([H])([H])C1C([H])=C([H])C([H])=C([H])C=1[H])=S
|
|
| InChi Key |
AEZGRQSLKVNPCI-UNOMPAQXSA-N
|
|
| InChi Code |
InChI=1S/C22H15NO4S2/c24-20-19(29-22(28)23(20)13-14-4-2-1-3-5-14)12-17-10-11-18(27-17)15-6-8-16(9-7-15)21(25)26/h1-12H,13H2,(H,25,26)/b19-12-
|
|
| 化学名 |
4-[5-[[4-Oxo-3-(phenylmethyl)-2-thioxo-5-thiazolidinylidene]methyl]-2-furanyl]-benzoic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~5 mg/mL ( 11.86 mM)
Water: Insoluble Ethanol: Insoluble |
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3725 mL | 11.8627 mL | 23.7254 mL | |
| 5 mM | 0.4745 mL | 2.3725 mL | 4.7451 mL | |
| 10 mM | 0.2373 mL | 1.1863 mL | 2.3725 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|