| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| Other Sizes |
|

| 靶点 |
Human Endogenous Metabolite
|
|---|---|
| 体外研究 (In Vitro) |
由于禁止化妆品动物实验,最近出现了用皮肤芯片(SOC)技术代替动物实验的各种尝试。之前,我们报道了一种无泵SOC的开发,该SOC能够通过一个简单的驱动器进行药物测试,该驱动器使用的原理是当芯片使用微流体通道倾斜时,介质通过重力沿通道流动。在这项研究中,我们使用无泵SOC,而不是在单细胞水平上进行药物测试,我们评估了α-硫辛酸/Lipoic acid (ALA),这是一种在皮肤等效物中被称为抗衰老物质,对皮肤组织和表皮结构形成的功效。比较和评价蛋白表达及基因分型的变化。苏木精和伊红染色组织学分析显示,在ALA存在或不存在的情况下,真皮层成纤维细胞的活性存在差异。我们观察到,在10 μM ALA处理下,随着培养时间的延长,表皮层逐渐突出。ALA的作用使聚丝蛋白、天青蛋白、角蛋白10和胶原IV的表皮结构蛋白表达增加。ALA处理后表皮层变化明显。由于衰老,对皮肤屏障功能和结构完整性的损害减少,表明ALA具有抗衰老作用。我们对ALA处理过的人皮肤等效物中的聚丝蛋白、天青蛋白、角蛋白10、整合蛋白和胶原蛋白I基因进行了基因分析,结果表明ALA处理后聚丝蛋白基因表达增加。这些结果表明,无泵SOC可作为类似人类皮肤的体外皮肤模型,并可分析其蛋白质和基因表达,未来可用于化妆品材料的功能药物测试。这项技术有望为皮肤病模型的发展做出贡献。[2]
|
| 体内研究 (In Vivo) |
硫辛酸/Lipoic acid (LA)和高压氧治疗(HBOT)可促进慢性伤口愈合。
目的:比较硫辛酸及其对映体R-(+)-硫辛酸(RLA)对创面愈合的影响。
材料与方法:LA + HBOT (L)组、RLA + HBOT (R)组和安慰剂+ HBOT (P)组。治疗前和第20、40天测量病变面积。在治疗前和第7、14天采集活检组织和血浆(测量血管内皮生长因子VEGF;EGF,表皮生长因子,TNF-α和IL-6)。
结果:RLA组溃疡改善明显。L组和R组EGF和VEFG均随时间升高。RLA降低了T7和T14的IL-6,而LA无此作用。LA和RLA的T14细胞TNF-α水平均降低。
讨论:伤口愈合的改善与EGF和VEGF的增加以及血浆TNF-α和IL-6的降低有关。
结论:RLA可能比LA更有效地改善HBO治疗患者的慢性伤口愈合。[1]
|
| 细胞实验 |
三维皮肤等效模型的构建[2]
如图10所示,将大鼠尾型胶原蛋白,10倍DMEM, 0.5 N NaOH,原代人成纤维细胞悬液(终细胞浓度5.0 × 105细胞/mL), 1倍DMEM混合,中和凝胶。大鼠尾胶原蛋白浓度固定为6.12 mg/mL。为了制造真皮层,我们将胶原蛋白- fbs悬浮液在芯片上播种至3mm高度,并在37°C的培养箱中,在5%的CO2下沉积40分钟。之后,真皮(DL)在DMEM(含10%胎牛血清和1%青霉素/链霉素)中培养5 d,每天更换培养基。将原代人角质形成细胞悬液(终细胞浓度1.0 × 106细胞/mL)接种于真皮层共培养2天。仅在DL-KCs上方提供KGM-GoldTM角质细胞生长培养基,并向芯片通道提供DMEM。硫辛酸/ALA提供了诱导KCs分化3 ~ 7天的电子培养基,同时通过将其暴露在空气中提供了与真实皮肤相似的环境(电子培养基组成:DMEM/Ham 's F12 (EGF-1 10 ng/mL,氢化可的松0.4 μg/mL,胰岛素5 μg/mL,转铁蛋白5 μg/mL, 3,3,5-三碘- l -甲状腺氨酸钠盐2 × 10−11 M,霍乱毒素10−10 M, 10% (v/v) FBS, 1%青霉素/链霉素)。ALA治疗在AE期(1 μM和10 μM ALA)进行。传代5-7使用人真皮FBs,传代4-6使用人表皮KCs。所有培养物在37°C的培养箱中,5% CO2下孵育。媒介每天都在变化。 |
| 动物实验 |
The patients were randomly divided into three groups: [1]
Group L: A total of 10 patients (four male and six female with a mean age of 59 (45–83) years old). There were total 13 ulcerated lesions: five caused by arterial impairment, four caused by venous insufficiency, three were diabetic/ischemic origin and one caused by trauma. The average ulcer size was 3.92 cm2 with a mean history of 217 d. The patients were treated with Lipoic acid/LA 600 mg orally 60 min before each session of HBOT. Group R: A total of 10 patients (five male and five female with a mean age of 71.8 (58–82) years old). There were total 10 ulcerated lesions: five caused by arterial impairment, four were diabetic/ischemic origin and one with diabetic neuropathy. The average ulcer size was 7.45 cm2 with a mean history of 233 d. These patients were treated with RLA, 600 mg orally 60 min before each session of HBOT. Group P: Seven patients (three male and four female with a mean age of 72.1 (49–88) years old). There were total seven ulcerated lesions: four were diabetic/ischemic origin, two caused by arterial impairment and one from venous insufficiency. The average ulcer size was 3.18 cm2 with a mean history 224 d. These patients were treated with placebo 60 min before each session of HBOT. |
| 药代性质 (ADME/PK) |
Metabolism / Metabolites
Paraoxonase (PON1) is a key enzyme in the metabolism of organophosphates. PON1 can inactivate some organophosphates through hydrolysis. PON1 hydrolyzes the active metabolites in several organophosphates insecticides as well as, nerve agents such as soman, sarin, and VX. The presence of PON1 polymorphisms causes there to be different enzyme levels and catalytic efficiency of this esterase, which in turn suggests that different individuals may be more susceptible to the toxic effect of OP exposure. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
(R)-lipoic acid is a cholinesterase or acetylcholinesterase (AChE) inhibitor. A cholinesterase inhibitor (or 'anticholinesterase') suppresses the action of acetylcholinesterase. Because of its essential function, chemicals that interfere with the action of acetylcholinesterase are potent neurotoxins, causing excessive salivation and eye-watering in low doses, followed by muscle spasms and ultimately death. Nerve gases and many substances used in insecticides have been shown to act by binding a serine in the active site of acetylcholine esterase, inhibiting the enzyme completely. Acetylcholine esterase breaks down the neurotransmitter acetylcholine, which is released at nerve and muscle junctions, in order to allow the muscle or organ to relax. The result of acetylcholine esterase inhibition is that acetylcholine builds up and continues to act so that any nerve impulses are continually transmitted and muscle contractions do not stop. Among the most common acetylcholinesterase inhibitors are phosphorus-based compounds, which are designed to bind to the active site of the enzyme. The structural requirements are a phosphorus atom bearing two lipophilic groups, a leaving group (such as a halide or thiocyanate), and a terminal oxygen. Health Effects Acute exposure to cholinesterase inhibitors can cause a cholinergic crisis characterized by severe nausea/vomiting, salivation, sweating, bradycardia, hypotension, collapse, and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved. Accumulation of ACh at motor nerves causes overstimulation of nicotinic expression at the neuromuscular junction. When this occurs symptoms such as muscle weakness, fatigue, muscle cramps, fasciculation, and paralysis can be seen. When there is an accumulation of ACh at autonomic ganglia this causes overstimulation of nicotinic expression in the sympathetic system. Symptoms associated with this are hypertension, and hypoglycemia. Overstimulation of nicotinic acetylcholine receptors in the central nervous system, due to accumulation of ACh, results in anxiety, headache, convulsions, ataxia, depression of respiration and circulation, tremor, general weakness, and potentially coma. When there is expression of muscarinic overstimulation due to excess acetylcholine at muscarinic acetylcholine receptors symptoms of visual disturbances, tightness in chest, wheezing due to bronchoconstriction, increased bronchial secretions, increased salivation, lacrimation, sweating, peristalsis, and urination can occur. Certain reproductive effects in fertility, growth, and development for males and females have been linked specifically to organophosphate pesticide exposure. Most of the research on reproductive effects has been conducted on farmers working with pesticides and insecticdes in rural areas. In females menstrual cycle disturbances, longer pregnancies, spontaneous abortions, stillbirths, and some developmental effects in offspring have been linked to organophosphate pesticide exposure. Prenatal exposure has been linked to impaired fetal growth and development. Neurotoxic effects have also been linked to poisoning with OP pesticides causing four neurotoxic effects in humans: cholinergic syndrome, intermediate syndrome, organophosphate-induced delayed polyneuropathy (OPIDP), and chronic organophosphate-induced neuropsychiatric disorder (COPIND). These syndromes result after acute and chronic exposure to OP pesticides. Symptoms Symptoms of low dose exposure include excessive salivation and eye-watering. Acute dose symptoms include severe nausea/vomiting, salivation, sweating, bradycardia, hypotension, collapse, and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved. Hypertension, hypoglycemia, anxiety, headache, tremor and ataxia may also result. Treatment If the compound has been ingested, rapid gastric lavage should be performed using 5% sodium bicarbonate. For skin contact, the skin should be washed with soap and water. If the compound has entered the eyes, they should be washed with large quantities of isotonic saline or water. In serious cases, atropine and/or pralidoxime should be administered. Anti-cholinergic drugs work to counteract the effects of excess acetylcholine and reactivate AChE. Atropine can be used as an antidote in conjunction with pralidoxime or other pyridinium oximes (such as trimedoxime or obidoxime), though the use of '-oximes' has been found to be of no benefit, or possibly harmful, in at least two meta-analyses. Atropine is a muscarinic antagonist, and thus blocks the action of acetylcholine peripherally. |
| 参考文献 |
|
| 其他信息 |
Pharmacodynamics
Lipoic acid (or α-lipoic acid) is able to pass the blood-brain barrier and is putatively used for detoxification of mercury attached to the brain cells. It can mobilise bound mercury into the blood stream as it is a mercaptan (sulfur compound which readily binds to the mercury). In the blood stream, another chelator such as dimercaptosuccinic acid (DMSA) or methylsulfonylmethane (MSM) is used to transfer mercury safely into the urine for excretion. Since DMSA cannot cross the blood-brain barrier, both lipoic acid and DMSA tend to be used together. It is hypothesized that this treatment-along with carnitine, dimethylglycine (DMG), Vitamin B6, folic acid, and magnesium—could be used to treat autism and amalgam poisoning. In this hypothesis, the reason why autism is difficult to treat is that mercury is attached to the brain cells and most medicines and vitamin supplements do not penetrate the blood-brain barrier. However, α-lipoic acid and perhaps vitamin B12 could making it possible for other chelators to remove mercury safely out of the body and could perhaps one day be used as a treatment for autism. Because lipoic acid is related to cellular uptake of glucose and it is both soluble in water and fat, it is being used for treatment in diabetes. It may be helpful for people with Alzheimer's disease or Parkinson's disease. Mechanism of Action: Lipoic Acid is generally involved in oxidative decarboxylations of keto acids and is presented as a growth factor for some organisms. Lipoic acid exists as two enantiomers, the R-enantiomer and the S-enantiomer. Normally only the R-enantiomer of an amino acid is biologically active, but for lipoic acid the S-enantiomer assists in the reduction of the R-enantiomer when a racemic mixture is given. Some recent studies have suggested that the S-enantiomer in fact has an inhibiting effect on the R-enantiomer, reducing its biological activity substantially and actually adding to oxidative stress rather than reducing it. Furthermore, the S-enantiomer has been found to reduce the expression of GLUT-4s in cells, responsible for glucose uptake, and hence reduce insulin sensitivity. (R)-lipoic acid is the (R)-enantiomer of lipoic acid. A vitamin-like, C8 thia fatty acid with anti-oxidant properties. It has a role as a prosthetic group, a nutraceutical and a cofactor. It is a lipoic acid, a member of dithiolanes, a heterocyclic fatty acid and a thia fatty acid. It is functionally related to an octanoic acid. It is a conjugate acid of a (R)-lipoate. It is an enantiomer of a (S)-lipoic acid. A vitamin-like antioxidant. Lipoic acid is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). (R)-lipoic acid is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Lipoic acid has been reported in Solanum lycopersicum with data available. Lipoic acid is a vitamin-like antioxidant that acts as a free-radical scavenger. Alpha-lipoic acid is also known as thioctic acid. It is a naturally occurring compound that is synthesized by both plants and animals. Lipoic acid contains two thiol groups which may be either oxidized or reduced. The reduced form is known as dihydrolipoic acid (DHLA). Lipoic acid (Delta E= -0.288) is therefore capable of thiol-disulfide exchange, giving it antioxidant activity. Lipoate is a critical cofactor for aerobic metabolism, participating in the transfer of acyl or methylamine groups via the 2-Oxoacid dehydrogenase (2-OADH) or alpha-ketoglutarate dehydrogenase complex. This enzyme catalyzes the conversion of alpha-ketoglutarate to succinyl CoA. This activity results in the catabolism of the branched chain amino acids (leucine, isoleucine and valine). Lipoic acid also participates in the glycine cleavage system(GCV). The glycine cleavage system is a multi-enzyme complex that catalyzes the oxidation of glycine to form 5,10 methylene tetrahydrofolate, an important cofactor in nucleic acid synthesis. Since Lipoic acid is an essential cofactor for many enzyme complexes, it is essential for aerobic life as we know it. This system is used by many organisms and plays a crucial role in the photosynthetic carbon cycle. Lipoic acid was first postulated to be an effective antioxidant when it was found it prevented vitamin C and vitamin E deficiency. It is able to scavenge reactive oxygen species and reduce other metabolites, such as glutathione or vitamins, maintaining a healthy cellular redox state. Lipoic acid has been shown in cell culture experiments to increase cellular uptake of glucose by recruiting the glucose transporter GLUT4 to the cell membrane, suggesting its use in diabetes. Studies of rat aging have suggested that the use of L-carnitine and lipoic acid results in improved memory performance and delayed structural mitochondrial decay. As a result, it may be helpful for people with Alzheimer's disease or Parkinson's disease. Lipoic acid is a metabolite found in or produced by Saccharomyces cerevisiae. An octanoic acid bridged with two sulfurs so that it is sometimes also called a pentanoic acid in some naming schemes. It is biosynthesized by cleavage of LINOLEIC ACID and is a coenzyme of oxoglutarate dehydrogenase (KETOGLUTARATE DEHYDROGENASE COMPLEX). It is used in DIETARY SUPPLEMENTS. |
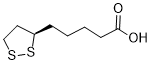
| 分子式 |
C8H14O2S2
|
|---|---|
| 分子量 |
206.3256
|
| 精确质量 |
206.043
|
| 元素分析 |
C, 46.57; H, 6.84; O, 15.51; S, 31.08
|
| CAS号 |
1200-22-2
|
| 相关CAS号 |
Lipoic acid; 1200-22-2; 1077-28-7 (racemate)
|
| PubChem CID |
6112
|
| 外观&性状 |
Light yellow to dark brown solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
362.5±11.0 °C at 760 mmHg
|
| 熔点 |
46-49ºC
|
| 闪点 |
173.0±19.3 °C
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
| 折射率 |
1.562
|
| LogP |
2.16
|
| tPSA |
87.9
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
12
|
| 分子复杂度/Complexity |
150
|
| 定义原子立体中心数目 |
1
|
| SMILES |
S1[C@@]([H])(C([H])([H])C([H])([H])S1)C([H])([H])C([H])([H])C([H])([H])C([H])([H])C(=O)O[H]
|
| InChi Key |
AGBQKNBQESQNJD-SSDOTTSWSA-N
|
| InChi Code |
InChI=1S/C8H14O2S2/c9-8(10)4-2-1-3-7-5-6-11-12-7/h7H,1-6H2,(H,9,10)/t7-/m1/s1
|
| 化学名 |
5-[(3R)-dithiolan-3-yl]pentanoic acid
|
| 别名 |
Thiogamma oral; R-(+)-alpha-Lipoic acid; (+)-alpha-Lipoic acid; Lipoate;Verla Lipon; Verla-Lipon; VerlaLipon; Lipoic Acid; Thioctacide T; Thioctic Acid; (R)-5-(1,2-Dithiolan-3-yl)pentanoic acid; Thiogamma Injekt; thioctic acid;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: (1). 本产品在运输和储存过程中需避光。 (2). 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ≥ 100 mg/mL (~484.7 mM)
H2O: ~1 mg/mL (~4.9 mM) |
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.8466 mL | 24.2330 mL | 48.4660 mL | |
| 5 mM | 0.9693 mL | 4.8466 mL | 9.6932 mL | |
| 10 mM | 0.4847 mL | 2.4233 mL | 4.8466 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03161028 | Active Recruiting |
Drug: Lipoic acid Drug: Placebo |
Multiple Sclerosis | VA Office of Research and Development |
July 1, 2018 | Phase 2 |
| NCT00765310 | Active Recruiting |
Dietary Supplement: Placebo Dietary Supplement: R-alpha lipoic acid |
Atherosclerosis | Oregon State University | April 2009 | Phase 2 Phase 3 |
| NCT00764270 | Active Recruiting |
Dietary Supplement: R-alpha lipoic acid |
Atherosclerosis | Oregon State University | August 2011 | Phase 2 Phase 3 |
| NCT02910531 | Active Recruiting |
Drug: Placebo Dietary Supplement: Alpha lipoic acid |
Cystinuria | Thomas Chi, MD | June 19, 2017 | Phase 2 |
| NCT06131918 | Active Recruiting |
Drug: Resveratrol Drug: Alpha lipoic acid |
Multiple Sclerosis | Khyber Medical University Peshawar | January 9, 2023 | Phase 2 |