| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 体外研究 (In Vitro) |
在 WM9、DU145、C4-2、Hey、WM480 和 A549 细胞中,喷他脒给药(0–10 µg/mL;6 天)以浓度依赖性方式减少癌细胞增殖 [1]。已经确定羟乙基磺酸喷他脒对婴儿利什曼原虫前鞭毛体具有细胞毒性。孵育 72 小时后,羟乙基磺酸喷他脒的杀利什曼病效果是顺铂的 60 倍。与顺铂相比,羟乙磺酸喷他脒会导致更大量的程序性细胞死亡 (PCD),这与 DNA 合成的抑制和细胞周期停滞在 G2/M 期有关。当喷他脒羟乙基磺酸盐与小牛胸腺 DNA (CT-DNA) 结合时,DNA 双螺旋会发生与 B 到 A 转变一致的结构变化。由于羟乙基磺酸喷他脒和泛素之间的相互作用,该蛋白质的 β-折叠组成增加了 6% [2]。
|
|---|---|
| 体内研究 (In Vivo) |
用喷他脒(0.25mg/只;肌内注射;每2天;持续4周)治疗无胸腺裸鼠可有效限制WM9人黑色素瘤的生长[1]。
|
| 细胞实验 |
细胞活力测定[1]
细胞类型: WM9、DU145、C4-2、Hey、WM480 和 A549 细胞 测试浓度: 0-10 µg /mL 孵育时间: 6 天 实验结果: 培养中所有六种细胞系的生长均以浓度依赖性方式受到抑制,生长完全抑制细胞系浓度为 10 µg/mL。 |
| 动物实验 |
Animal/Disease Models: Athymic nude mice (6 weeks old) injected with WM9 cells [1]
Doses: 0.25mg/mouse Route of Administration: intramuscularinjection; once every 2 days; for 4 consecutive weeks Experimental Results: Dramatically inhibited WM9 human melanoma Growth in nude mice. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorbed poorly through the gastrointestinal tract and is usually administered parenterally. Pentamidine isethionate is fairly well absorbed from parenteral sites of admin despite the formation of sterile abscesses that may occur after its used. Following a single intravenous dose, the drug disappears from plasma with an apparent half-life of several min to a few hours; this is followed by a slower distribution phase and a prolonged elimination phase lasting from weeks to months. Patients with African trypanosomiasis exhibit marked interindividual variations in pharmacokinetic parameters. Their mean system plasma clearance after a single dose is about 1120 mL/min, but the volume of distribution is about 25,000 L, a finding that accounts for the prolonged average elimination half-life of about 12 days ... The renal clearance of pentamidine averages only about 2% to 11% of its systemic clearance ... but whether the drug is metabolized or excreted in bile ... is unknown. In patients receiving multiple injections of the drug over a 13-day period for treatment of pneumocystosis, drug accumulation occurs such that no steady-state plasma concn is attained ... /Pentamidine isethionate/ ... After multiple parenteral doses, the liver, kidney, adrenal, and spleen of patients with AIDS contain the highest concn of drug, whereas only traces are found in the brain ... Lungs of such patients contain intermediate but therapeutic concn after 5 daily doses of 4 mg of base/kg. Higher pulmonary concn should be achieved by inhalation of pentamidine aerosols for prophylaxis or as adjunctive treatment for mild to moderate Pneumocystis carinii pneumonia; delivery of drug by this route results in little systemic absorption and decreased toxicity compared with intravenous admin in both adults and children. The actual dose delivered to the lungs depends on both the size of particles generated by the nebulizer and the patient's ventilatory patterns. /Pentamidine isethionate/ Aerosolized pentamidine produces concentrations approximately 10 to 100 times higher in the lungs than would a comparable dose of IV pentamidine. Systemic absorption of inhaled pentamidine is minimal, with serum pentamidine concentrations less than 20 nanograms per mL after a nebulized dose of 4 mg/kg in most cases (versus 612 nanogram per mL after a single IV dose of 4 mg/kg). Peak systemic absorption occurs at, or near, completion of inhalation therapy. For more Absorption, Distribution and Excretion (Complete) data for PENTAMIDINE (14 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic. By using high-performance liquid chromatography, the in vitro conversion of pentamidine to the corresponding amidoximes (N-hydroxypentamidine and N,N'-dihydroxypentamidine) was studied in supernatants of rat liver homogenate centrifuged at 9,000 x g. The presence of the two amidoxime peaks in chromatograms was confirmed by liquid secondary ion mass spectrometry and by unequivocal synthesis of the suspected metabolites. The metabolic reactions were found to be catalyzed by the cytochrome P-450 system (mixed-function oxidases). The formation of the monohydroxylated product was found to have a Km of 0.48 mM and a Vmax of 29.50 pmol/min per mg of protein, while the dihydroxylated metabolite had a Km of 0.73 mM and a Vmax of 4.10 pmol/min per mg of protein. ... The antiprotozoal/antifungal drug pentamidine [1,5-bis(4-amidinophenoxy)pentane] has been recently shown to be metabolized by rat liver fractions to at least six putative metabolites as detected by high-performance liquid chromatography. ... In this study, the two major microsomal metabolites have been identified as the 2-pentanol and 3-pentanol analogs of pentamidine [1,5-di(4-amidinophenoxy)-2-pentanol; and 1,5-bis(4-amidinophenoxy)-3-pentanol]. As well, a seventh putative metabolite has been discovered and identified as para-hydroxybenzamidine, a fragment of the original drug. ... the cytochromes P-450 have been demonstrated as the enzyme system responsible for pentamidine metabolism ... the mixed-function oxidases readily convert pentamidine to hydroxylated metabolites, but exactly which isozyme(s) of cytochrome P-450 is responsible is not clear. The antiprotozoal drug pentamidine [1,5-bis(4'-amidinophenoxy)pentane] has been previously shown to be metabolized by rat liver microsomes, and five of the seven putative primary metabolites have been identified. With the synthesis and identification of 5-(4'-amidinophenoxy)pentanoic acid and 5-(4'-amidinophenoxy)-1-pentanol as the remaining two metabolites, the primary metabolism of pentamidine in rats appears fully characterized. ... Isolated, perfused rat livers were used with [14C]pentamidine to identify secondary metabolites. Only two novel radioactive peaks were detected by HPLC analysis of perfused liver samples. The treatment of liver samples with sulfatase or beta-glucuronidase resulted in the reduction or elimination of these peaks and gave rise to peaks identified as para-hydroxybenzamidine and 5-(4'-amidinophenoxy)pentanoic acid. It was concluded from these results that only these two primary metabolites were conjugated with sulfate or glucuronic acid. Pentamidine /is a substrate for/ human liver microsomal P450 enzyme CYP2C19. /From table/ Hepatic. Half Life: 9.1-13.2 hours Biological Half-Life 9.1-13.2 hours Intramuscular: 9.1 to 13.2 hours. Intravenous: Approximately 6.5 hours. Terminal half-life: 2 to 4 weeks. Renal function impairment: Pentamidine half-life may be prolonged in patients with renal dysfunction ; however, no correlation between renal function and plasma clearance of pentamidine has been found. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
The mode of action of pentamidine is not fully understood. It is thought that the drug interferes with nuclear metabolism producing inhibition of the synthesis of DNA, RNA, phospholipids, and proteins. Hepatotoxicity Pentamidine has been associated with serum aminotransferase elevations in 9% to 15% of patients receiving 2 to 3 weeks of therapy for pneumocystis pneumonia. Clinically apparent liver injury has also been reported with its use, but always in association with multiple other severe complications, such as respiratory or renal failure and pancreatitis. The onset of injury is within days of starting therapy and is characterized by acute hepatic necrosis, marked elevations in serum aminotransferase levels, rapid development of prolongation of prothrombin time and minimal or no jaundice. Recovery is typically rapid and usually complete. Likelihood score: D (possible rare cause of clinically apparent liver injury). Protein Binding 69% Interactions Since nephrotoxic effects may be additive, the concurrent or sequential use of pentamidine isethionate and other drugs with similar toxic potentials such as aminoglycosides, amphotericin B, capreomycin, colistin, cisplatin, foscarnet, methoxyflurane, polymyxin B, or vancomycin should be closely monitored or avoided, if possible. Concurrent use of other nephrotoxic medications with pentamidine may increase the potential for nephrotoxicity; renal function determinations, dosage reductions, and/or dosage interval adjustments may be required. Renal side-effects are frequently observed after parenteral administration of pentamidine. In this study in a rat model, the nephrotoxicity was assessed by measuring urinary loss of tubular cells, malate dehydrogenase activity and creatinine clearance. In addition, we studied the influence of other nephrotoxins such as tobramycin, amphotericin B and cyclosporin on the pentamidine-associated nephrotoxicity and proved the possibilities of reducing this toxicity by coadministration with other drugs. The tubular toxicity of pentamidine (1, 10 or 20 mg/kg daily) is dose-related and reversible. The toxicity can be reduced by coadministration of fosfomycin (1 x 500 or 2 x 250 mg/kg daily) and D-glucaro-1,5-lactam (2 x 5 mg/kg daily) and enhanced by tobramycin (2 x 2.5 mg/kg daily), amphotericin B (1 mg/kg daily) and cyclosporin (10 mg/kg daily). Furthermore, an increase in the creatinine clearance in pentamidine-treated rats can be obtained with both verapamil (2 x 1.5 mg/kg daily) and enalapril (5 mg/kg daily). Concurrent use /of foscarnet/ with pentamidine may result in severe, but reversible, hypocalcemia, hypomagnesemia, and nephrotoxicity. For more Interactions (Complete) data for PENTAMIDINE (8 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse subcutaneous 120 mg/kg LD50 Mouse intraperitoneal 63 mg/kg LD50 Mouse IV 15 mg/kg |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Pentamidine is indicated in the treatment of Pneumocystis carinii pneumonia (PCP) in immunocompromised patients, including patients with acquired immunodeficiency syndrome (AIDS). Sulfamethoxazole and trimethoprim combination is considered to be the primary agent for PCP in patients who can tolerate it. /Included in US product labeling/ Pentamidine is used as a secondary agent in the treatment of visceral leishmaniasis (kala-azar) caused by Leishmania donovani. Stibogluconate sodium, a pentavalent antimony derivative, is considered to be the primary agent for visceral leishmaniasis. /NOT included in US product labeling/ Pentamidine is used as a secondary agent in the treatment of cutaneous leishmaniasis caused by Leishmania tropica, L. major, L. mexicana, L. aethiopica, L. peruviana, L. guyanensis, and L. braziliensis. Stibogluconate sodium, a pentavalent antimony derivative, is considered to be the primary agent for cutaneous leishmaniasis. /NOT included in US product labeling/ Aerosolized pentamidine is indicated in both secondary prophylaxis (patients who have already had at least one episode of Pneumocystis carinii pneumonia) and primary prophylaxis (HIV-infected patient with a CD4 lymphocyte count less than or equal to 200 cells per cubic millimeter) of Pneumocystis carinii pneumonia. /Included in US product labeling/ For more Therapeutic Uses (Complete) data for PENTAMIDINE (12 total), please visit the HSDB record page. Drug Warnings Fatalities due to severe hypotension, hypoglycemia, acute pancreatitis and cardiac arrhythmias have been reported in patients treated with pentamidine isethionate, both by the IM and IV routes. Severe hypotension may result after a single IM or IV dose and is more likely with rapid IV administration. The administration of the drug should, therefore, be limited to the patients in whom Pneumocystis carinii has been demonstrated. Patients should be closely monitored for the development of serious adverse reactions. Nephrotoxicity reportedly occurs in at least 25% of patients with pneumocystis pneumonia receiving parenteral pentamidine isethionate. Pentamidine-induced nephrotoxicity is manifested by an increase in serum creatinine concentration and/or BUN, usually developing gradually and appearing during the second week of therapy with the drug. Azotemia also has been reported. Renal insufficiency is usually mild to moderate in severity and reversible following discontinuance of pentamidine; however, acute renal failure (e.g., serum creatinine concentration greater than 6 mg/dL) or severe renal insufficiency requiring discontinuance of the drug may occur occasionally. Limited evidence suggests that nephrotoxicity and hyperkalemia both may occur more frequently in patients with AIDS than in other patients treated with parenteral pentamidine; hyperkalemia has been severe in some patients. Rarely, pentamidine-induced acute renal failure has been associated with myoglobinuria or gross hematuria. The risk and degree of pentamidine-induced renal impairment may be increased in the presence of dehydration or by concomitant use of other nephrotoxic drugs. Acute renal failure has been reported in at least 1 patient receiving pentamidine inhalation therapy; flank pain and nephritis also have been reported occasionally in patients receiving the aerosolized drug by oral inhalation via nebulizer. Cardiorespiratory arrest (following rapid IV injection), ventricular tachycardia, atypical ventricular tachycardia (torsade de pointes), ECG abnormalities, and facial flushing have also been reported in patients receiving parenteral pentamidine. The risk of hypotensive reactions following IM or IV administration of pentamidine isethionate has not been directly compared, but some data suggest that there is no difference in the frequency of these reactions following either route of administration when IV infusions of the drug are administered over a period of at least 60 minutes. Hypotensive reactions may be particularly likely to occur following rapid IV injection or infusion. To minimize the risk of this adverse effect when pentamidine isethionate is administered IV, infusions of the drug should be given over a period of 60-120 minutes. However, hypotension, which was not ameliorated by adjustment of the infusion rate, persisted beyond completion of the infusion, and required volume expansion for correction, has been reported in some patients. Hypotension, hypertension, tachycardia, palpitations, syncope, dizziness, light-headedness, diaphoresis, cerebrovascular accident, vasodilatation, and vasculitis have been reported occasionally in patients receiving orally inhaled pentamidine. Since pentamidine has become commercially available, there is renewed interest in using it as the initial treatment for Pneumocystis carinii pneumonia in AIDS patients. /The authors/ reviewed the use of pentamidine in 24 patients with Pneumocystis carinii pneumonia to gain information on the prevalence and severity of adverse effects from this drug. Twenty out of twenty-four patients (83 percent) experienced some kind of adverse effect. Hepatic abnormalities (58 percent), nausea and vomiting (46 percent), hypoglycemia (33 percent), azotemia (25 percent), and pain at the injection site (25 percent) were the most frequently seen effects. For more Drug Warnings (Complete) data for PENTAMIDINE (25 total), please visit the HSDB record page. Pharmacodynamics Pentamidine is an antiprotozoal agent. It is an aromatic diamidine, and is known to have activity against Pneumocystis carinii. The exact nature of its antiprotozoal action is unknown. in vitro studies with mammalian tissues and the protozoan Crithidia oncopelti indicate that the drug interferes with nuclear metabolism producing inhibition of the synthesis of DNA, RNA, phospholipids and proteins. Little is known about the drug's pharmacokinetics. The medication is also useful in Leishmaniasis and in prophylaxis against sleeping sickness caused by Trypanosoma brucei gambiense. Hydration before treatment lessens the incidence and severity of side effects, which include liver or kidney dysfunction, hypertension, hypotension, hypoglycemia, hypocalemia, leukopenia, thrombcytopenia, anemia, and allergic reaction. It is generally well-tolerated. |
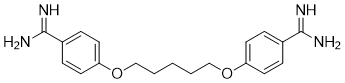
| 分子式 |
C19H24N4O2
|
|---|---|
| 分子量 |
340.41946
|
| 精确质量 |
340.189
|
| CAS号 |
100-33-4
|
| 相关CAS号 |
Pentamidine isethionate;140-64-7;Pentamidine dihydrochloride;50357-45-4;Pentamidine dimesylate;6823-79-6
|
| PubChem CID |
4735
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
539.4±60.0 °C at 760 mmHg
|
| 熔点 |
186ºC (dec.)
|
| 闪点 |
280.0±32.9 °C
|
| 蒸汽压 |
0.0±1.4 mmHg at 25°C
|
| 折射率 |
1.593
|
| LogP |
2.47
|
| tPSA |
118.2
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
25
|
| 分子复杂度/Complexity |
376
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
XDRYMKDFEDOLFX-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H24N4O2/c20-18(21)14-4-8-16(9-5-14)24-12-2-1-3-13-25-17-10-6-15(7-11-17)19(22)23/h4-11H,1-3,12-13H2,(H3,20,21)(H3,22,23)
|
| 化学名 |
4-[5-(4-carbamimidoylphenoxy)pentoxy]benzenecarboximidamide
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9375 mL | 14.6877 mL | 29.3755 mL | |
| 5 mM | 0.5875 mL | 2.9375 mL | 5.8751 mL | |
| 10 mM | 0.2938 mL | 1.4688 mL | 2.9375 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。