| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
PHENFORMIN IS ADEQUATELY ABSORBED FROM GI TRACT. DRUG HAS SHORT T/2 (3 HR) & CORRESPONDINGLY BRIEF DURATION OF ACTION. HYPOGLYCEMIC EFFECT MAY BE PROLONGED TO BETWEEN 6 & 14 HR WITH USE OF TIMED-DISINTEGRATION CAPSULES. (14)C-LABELED PHENFORMIN ADMIN TO RATS (100 MG/KG ORALLY OR IP) & GUINEA PIGS (25 MG/KG ORALLY & 12.5 IP). EXCRETION OF RADIOACTIVITY & METAB WAS SLOWER IN GUINEA PIGS WHICH MAY PARTLY EXPLAIN THE INCR PHARMACOLOGICAL RESPONSE OF GUINEA PIGS TO PHENFORMIN. RATS ELIMINATED 26% OF AN INTRADUODENAL DOSE OF LABELED PHENFORMIN (20 MG/KG) IN BILE IN 6 HR COMPARED TO 6% IN GUINEA PIG. IN 8 DIABETIC PT HALF-LIFE OF PHENFORMIN WAS UNRELATED TO DEGREE OF RENAL IMPAIRMENT, WHEREAS REDUCED RENAL CLEARANCES OF INSULIN & CREATININE WERE SIGNIFICANTLY CORRELATED WITH PROLONGED HALF-LIFE OF ITS METABOLITE P-HYDROXYPHENETHYLBIGUANIDE. Metabolism / Metabolites IN RATS & GUINEA PIGS, MAJOR METABOLITE OF PHENFORMIN, N(1)-BETA-PHENETHYLBIGUANIDE, IS N(1)-P-HYDROXY-BETA-PHENETHYLBIGUANIDE, & CORRESPONDING O-ETHER GLUCURONIDE HAS ALSO BEEN DETECTED. METAB IN RATS & GUINEA PIGS. RATS EXCRETED LARGE AMT OF 4-HYDROXYPHENFORMIN (FREE & GLUCURONIC ACID CONJUGATED) & SOME UNCHANGED PHENFORMIN. METAB VARIED WITH DOSE & ROUTE OF ADMIN. GUINEA PIGS EXCRETED SMALL AMT OF 4-HYDROXYPHENFORMIN AFTER IP ADMIN & NONE AFTER ORAL ADMIN. LABELED COMPD WAS ADMIN. AN UNIDENTIFIED METAB & ITS GLUCURONIDE, WHICH MAY RESULT FROM ALIPHATIC C- OR N-HYDROXYLATION, ACCOUNTED FOR 47% OF 24-HR URINARY RADIOACTIVITY (17% OF DOSE) FOLLOWING ORAL ADMIN TO GUINEA PIGS. 26 HR FOLLOWING ADMIN OF SINGLE DOSE OF PHENFORMIN, 50 MG/KG ORALLY, P-HYDROXYPHENFORMIN WAS MAJOR URINARY METAB IN PHENOTYPICALLY EXTENSIVE METABOLIZERS, BUT WAS NOT OBSERVED IN PHENOTYPICALLY POOR METABOLIZERS. METAB IN 8 DIABETIC PT WITH RENAL IMPAIRMENT. EXCRETION OF THE METAB P-HYDROXYPHENETHYLBIGUANIDE WAS VARIABLE (BETWEEN 4.9% & 27% OF TOTAL URINARY DOSE LOSS) PROBABLY DUE TO GENETIC POLYMORPHISM OF HEPATIC MECHANISMS FOR HYDROXYLATION. Phenformin has known human metabolites that include p-Hydroxyphenylethylbiguanide. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
PHENFORMIN HAS BEEN REPORTED...TO ENHANCE ACTIVITY OF WARFARIN. PROPOSED MECHANISM IS INCR FIBRINOLYTIC EFFECT CAUSED BY PHENFORMIN SEEN DURING FIRST FEW MO OF TREATMENT. USE OF PROPRANOLOL IN DIABETIC PT...CAN RESULT IN DISTURBANCE OF CARBOHYDRATE METABOLISM & SHOULD BE AVOIDED. IF INSULIN & PROPRANOLOL...GIVEN CONCURRENTLY, PERIODIC SERUM GLUCOSE LEVELS SHOULD BE DETERMINED. ...SIMILAR PRECAUTIONS...APPLICABLE TO CONCURRENT USE OF...PHENFORMIN. DIABETIC PT TREATED WITH PHENFORMIN SHOULD AVOID INGESTION OF ALCOHOLIC BEVERAGES BECAUSE CONCURRENT USE MAY CAUSE HYPOGLYCEMIC REACTIONS OR LEAD TO LIFE-THREATENING LACTIC ACIDOSIS WITH SHOCK. DIPHENYLHYDANTOIN GIVEN IP TO RATS DECR LIVER LEVELS OF THIAMIN, RIBOFLAVIN, NIACIN, & PANTOTHENIC ACID. HEPATIC THIAMIN CONTENT WAS NORMALIZED BY SIMULTANEOUS ADMIN OF EITHER ACETOHEXAMINE OR PHENFORMIN. For more Interactions (Complete) data for PHENFORMIN (6 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
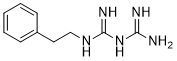
Phenformin is a member of the class of biguanides that is biguanide in which one of the terminal nitrogen atoms is substituted by a 2-phenylethyl group. It was used as an anti-diabetic drug but was later withdrawn from the market due to potential risk of lactic acidosis. It has a role as an antineoplastic agent, a geroprotector and a hypoglycemic agent. It is functionally related to a biguanide.
A biguanide hypoglycemic agent with actions and uses similar to those of metformin. Although it is generally considered to be associated with an unacceptably high incidence of lactic acidosis, often fatal, it is still available in some countries. (From Martindale, The Extra Pharmacopoeia, 30th ed, p290) Phenformin is an agent belonging to the biguanide class of antidiabetics with antihyperglycemic activity. Phenformin is not used clinically due to the high risk of lactic acidosis that is associated with its use. A biguanide hypoglycemic agent with actions and uses similar to those of METFORMIN. Although it is generally considered to be associated with an unacceptably high incidence of lactic acidosis, often fatal, it is still available in some countries. (From Martindale, The Extra Pharmacopoeia, 30th ed, p290) Drug Indication For the reatment of type II diabetes mellitus. Mechanism of Action Phenformin binds to the AMP-activated protein kinase (AMPK). AMPK is an ultra-sensitive cellular energy sensor that monitors energy consumption and down-regulates ATP-consuming processes when activated. The biguanide phenformin has been shown to independently decrease ion transport processes, influence cellular metabolism and activate AMPK. Phenformin's hypoglycemic activity is related the effect it has in activating AMPK and fooling insulin sensitive cells into thinking that insulin levels are low and causing the body to use glucose as if in a state of low caloric consumption. This drug also seems to inhibit several varients of ATP-sensitive potassium channels (namely the receptor subtype Kir6.1). IN VITRO, PHENFORMIN, IN RELATIVELY LARGE DOSES, INCR GLUCOSE UTILIZATION BY ENHANCING ANAEROBIC GLYCOLYSIS. THIS IS THOUGHT TO OCCUR AS RESULT OF, OR COINCIDENT WITH, INHIBITION OF CELLULAR RESPIRATION. ...ADENOSINE TRIPHOSPHATE (ATP) CONCN FALL & THOSE OF LACTATE INCR. SECOND ACTION OF DRUG IS TO DECR GLUCONEOGENESIS. ...MOST RECENTLY RECOGNIZED IS INHIBITION OF INTESTINAL ABSORPTION OF GLUCOSE & PROBABLY CERTAIN OTHER SUBSTANCES AS WELL; FOR EXAMPLE, DECR ABSORPTION OF VITAMIN B12 HAS BEEN OBSERVED. ...DOES NOT ACT IN NORMAL SUBJECT...PRESUMABLY BECAUSE INCR IN PERIPHERAL GLUCOSE UTILIZATION IS COMPENSATED FOR BY INCR HEPATIC GLUCOSE... BIGUANIDES APPARENTLY LOWER BLOOD SUGAR INDIRECTLY BY INHIBITING GLUCONEOGENESIS & INCR INSULIN SENSITIVITY. /ORAL HYPOGLYCEMICS/ They induce and increase in peripheral glucose utilization, a decrease in hepatic gluconeogenesis, and a decrease in intestinal absorption of glucose, vitamin B, and bile acids. /Biguanides/ Phenformin generally lowers the blood sugar only in the diabetic patient; it also depresses the blood sugar level in a nutritionally starved individual but not in one who is well fed. In its usual dose administered to a healthy individual, phenformin does not induce lactic acidosis. Phenformin requires insulin for its action, but does not induce and elevation in plasma insulin levels. Therapeutic Uses Hypoglycemic Agents EXPTL USE: PHENFORMIN (2 MG) ADMIN 5 DAYS/WK TO C3H/SN MICE FROM AGE 3.5 MO UNTIL DEATH DECR THE NUMBER OF SPONTANEOUS TUMORS 4.0 FOLD & AVG SURVIVAL OF ANIMALS BY 100 DAYS. IF PT REQUIRES MORE THAN 40 UNITS OF INSULIN/DAY, HE IS UNLIKELY TO RESPOND TO PHENFORMIN. ...PHENFORMIN PLUS ESTROGENS HAVE BEEN USED WITH SUCCESS IN REDUCING MORTALITY IN SURVIVORS OF MYOCARDIAL INFARCTION. PHENFORMIN IS USED IN TREATMENT OF MATURITY-ONSET DIABETES... For more Therapeutic Uses (Complete) data for PHENFORMIN (8 total), please visit the HSDB record page. Drug Warnings IN PRESENCE OF RENAL GLYCOSURIA, FATAL HYPOGLYCEMIA CAN OCCUR. IRREVERSIBLE LACTIC ACIDOSIS OCCURRED IN TWO PATIENTS UNDERGOING PHENFORMIN THERAPY FOR DIABETES. PHENFORMIN...ANTIDIABETIC AGENT TAKEN ORALLY, IS REPORTED TO HAVE CAUSED TRANSITORY MYOPIA IN 53-YR-OLD DIABETIC PATIENTS. DIABETIC SUBJECTS WITH SEVERE HEPATIC OR RENAL INSUFFICIENCY OR CONGESTIVE HEART FAILURE ARE NOT SUITABLE CANDIDATES FOR ORAL HYPOGLYCEMIC THERAPY. ...ITS ADMIN DURING PREGNANCY IS CURRENTLY NOT RECOMMENDED. For more Drug Warnings (Complete) data for PHENFORMIN (11 total), please visit the HSDB record page. Pharmacodynamics Used to treat diabetes, phenformin is a biguanide (contains 2 guanidino groups) hypoglycemic agent with actions and uses similar to those of metformin (Glucophage). Both drugs work by (1) decreasing the absorption of glucose by the intestines, (2) decreasing the production of glucose in the liver, and by (3) increasing the body's ability to use insulin more effectively. More specifically, phenformin improves glycemic control by improving insulin sensitivity. Phenformin is generally considered to be associated with an unacceptably high incidence of actic acidosis. In general biguanides should be used only in stable type II diabetics who are free of liver, kidney and cardiovascular problems and who cannot be controlled with diet. |
| 分子式 |
C10H15N5
|
|---|---|
| 分子量 |
205.27
|
| 精确质量 |
205.132
|
| CAS号 |
114-86-3
|
| 相关CAS号 |
Phenformin hydrochloride;834-28-6
|
| PubChem CID |
8249
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
332.2±35.0 °C at 760 mmHg
|
| 熔点 |
280-282°C
|
| 闪点 |
154.7±25.9 °C
|
| 蒸汽压 |
0.0±0.7 mmHg at 25°C
|
| 折射率 |
1.620
|
| LogP |
-0.6
|
| tPSA |
97.78
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
15
|
| 分子复杂度/Complexity |
236
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
ICFJFFQQTFMIBG-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C10H15N5/c11-9(12)15-10(13)14-7-6-8-4-2-1-3-5-8/h1-5H,6-7H2,(H6,11,12,13,14,15)
|
| 化学名 |
1-(diaminomethylidene)-2-(2-phenylethyl)guanidine
|
| 别名 |
BRN 1977317 Azucaps DebeonePhenformin Insoral
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.8716 mL | 24.3582 mL | 48.7163 mL | |
| 5 mM | 0.9743 mL | 4.8716 mL | 9.7433 mL | |
| 10 mM | 0.4872 mL | 2.4358 mL | 4.8716 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。