| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 体内研究 (In Vivo) |
暴露于对氧磷引起的呼吸道毒性的小鼠可以通过单次肌肉注射碘解磷定(10-150 mg/kg)来恢复[3]。
|
|---|---|
| 动物实验 |
Animal/Disease Models: Male F1B6D2 mice (diethyl paraoxon is toxic but not lethal in conscious, unrestrained mice) [3]
Doses: 10, 50, 100 and 150 mg/kg Route of Administration: Results of a single intramuscularinjection: induced partial (albeit complete) reversal of respiratory toxicity at a dose of 50 mg/kg, and complete reversal of diethyl paraoxon-induced respiratory toxicity in mice at a dose of 150 mg/kg. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The drug is rapidly excreted in the urine partly unchanged, and partly as a metabolite produced by the liver. It is not known if pralidoxime crosses the human placenta to the embryo or fetus. Pralidoxime chloride is a quaternary ammonium compound, but the molecular weight of the free base (about 137) is low enough for passage across the placenta. The rapid elimination of the drug should mitigate this transfer. The specific mechanism by which the renal tubule handles pralidoxime, a quaternary ammonium compound used to reactivate organophosphate-inhibited cholinesterase, has been studied using 22 subjects. Each subject was placed under certain conditions in the course of the study. All 22 received pralidoxime (5 mg/kg, IV, over a 2-min interval) under conditions of forced hydration and bed rest to serve as controls. Eight subjects received pralidoxime under conditions of forced hydration and bed rest, one time after 36 hr of ammonium chloride acidification, and another time after sodium bicarbonate alkalinization. Nine subjects received pralidoxime under forced dehydration and bed rest, 20-30 min after thiamine (200 mg total, IM), organic base. Eight received pralidoxime under forced hydration and bed rest simultaneously with p-aminohippurate (900 mg total, IV), organic acid. Four received pralidoxime under bed rest, after 8-12 hr of fasting, NPO. The drug is rapidly cleared from the plasma by renal tubular secretion. Reduction of pralidoxime clearance rates and prolongation of the biologic half-life after thiamine administration as compared to those after PAH administration suggest that pralidoxime is secreted as an organic base. Reduction of the excretion of pralidoxime under conditions of both urine alkalinization and urine acidification implicates an active reabsorption of pralidoxime not heretofore described. The pharmacokinetics of pralidoxime chloride (2-PAM) was studied in rats. Different groups of rats were given an intramuscular injection of 2-PAM at one of three doses (20, 40, or 80 mg/kg). This range of doses is used commonly in studies concerned with the efficacy of 2-PAM against poisoning by potent organophosphorus inhibitors of cholinesterase enzyme. Individual, sequential blood samples were collected during the course of the experiment. From these blood samples the plasma concentrations of 2-PAM were determined over time for each animal. Next the relationship of plasma concentration to time was expressed in terms of a standard pharmacokinetic model. Estimates of various pharmacokinetic parameters were calculated using an open, one-compartment model: volume of distribution (Vd), maximal plasma concentration (Cmax), elimination rate constant (k10), absorption rate constant (k01), area under the curve (AUC) and clearance (CL). Of the pharmacokinetic estimates, only Cmax and AUC were found to be statistically significant (p less than 0.0001) when compared across all the doses; these pharmacokinetic estimates were highly correlated with doses with r = 0.998 and r = 0.997, respectively. However, when AUC and Cmax were normalized by dividing through by dose, no significant differences were found in the transformed data. The results of this study in rat indicate that the pharmacokinetics of 2-PAM is linearly related to dose in a range employed in therapeutic studies of 2-PAM. BACKGROUND: Current therapies for organophosphate poisoning involve administration of oximes, such as pralidoxime (2-PAM), that reactivate the enzyme acetylcholinesterase. Studies in animal models have shown a low concentration in the brain following systemic injection. METHODS: To assess 2-PAM transport, we studied transwell permeability in three Madin-Darby canine kidney (MDCKII) cell lines and stem cell-derived human brain microvascular endothelial cells (BC1-hBMECs). To determine whether 2-PAM is a substrate for common brain efflux pumps, experiments were performed in the MDCKII-MDR1 cell line, transfected to overexpress the P-gp efflux pump, and the MDCKII-FLuc-ABCG2 cell line, transfected to overexpress the BCRP efflux pump. To determine how transcellular transport influences enzyme reactivation, we developed a modified transwell assay where the inhibited acetylcholinesterase enzyme, substrate, and reporter are introduced into the basolateral chamber. Enzymatic activity was inhibited using paraoxon and parathion. RESULTS: The permeability of 2-PAM is about 2 x 10(-6) cm/s in MDCK cells and about 1 x 10(-6) cm/s in BC1-hBMECs. Permeability is not influenced by pre-treatment with atropine. In addition, 2-PAM is not a substrate for the P-gp or BCRP efflux pumps. CONCLUSIONS: The low permeability explains poor brain penetration of 2-PAM and therefore the slow enzyme reactivation. This elucidates one of the reasons for the necessity of sustained intravascular (IV) infusion in response to organophosphate poisoning. For more Absorption, Distribution and Excretion (Complete) data for 2-PAM (10 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic Although the exact metabolic fate of pralidoxime has not been completely elucidated, the drug is believed to be metabolized in the liver. ... A recent study has suggested that active tubular secretion may be involved, although the specific mechanism has not been identified. There is a trend towards increasing doses of pralidoxime to treat human organophosphate poisonings that may have relevance in subpopulations. Indeed, pralidoxime is eliminated unchanged by the renal route. This study assesses the effect of renal failure on the kinetics of pralidoxime in a rat model of acute renal failure induced by potassium dichromate administration. On the first day, Sprague-Dawley rats received subcutaneously potassium dichromate (study) or saline (control). Forty-eight hours post-injection, animals received pralidoxime methylsulfate (50 mg/kg of pralidoxime base) intramuscularly. Blood specimens were sampled during 180 min after the injection. Urine was collected daily during the 3 days of the study. Plasma pralidoxime concentrations were measured by liquid chromatography with electrochemical detection. There was a 2-fold increase in mean elimination half-life and a 2.5-fold increase in mean area under the curve in the study compared to the control group. The mean total body clearance was halved in the study compared to the control group. Our study showed acute renal failure does not modify the distribution of pralidoxime but significantly alters its elimination from plasma. These results suggest that dosages of pralidoxime should be adjusted in organophosphate-poisoned humans with renal failure when using high dosage regimen of pralidoxime. Biological Half-Life 74-77 minutes The half-life of pralidoxime in patients with normal renal function varies and has been reported to range from 0.8-2.7 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Pralidoxime is an antidote and cholinesterase reactivator used in the treatment of poisoning due to pesticides and chemicals which have anticholinesterase activity. It is also used to treatment overdoses by anticholinesterase drugs used in the treatment of myasthenia gravis. Pralidoxime chloride is used concomitantly with atropine for the treatment of nerve agent poisoning in the context of chemical warfare or terrorism. Pralidoxime chloride must be administered within minutes to hours following exposure to nerve agents to be effective. HUMAN STUDIES: Manifestations of overdosage in normal subjects include dizziness, blurred vision, diplopia, headache, impaired accommodation, nausea, and slight tachycardia. In therapy, it has been difficult to differentiate side effects due to the drug from those due to the effects of the poison. When atropine and pralidoxime chloride are used together, the signs of atropinization (flushing, mydriasis, tachycardia, dryness of the mouth and nose) may occur earlier than might be expected when atropine is used alone. ANIMAL STUDIES: Pralidoxime, used in the treatment of organophosphate poisoning, significantly increased cardiac output at all doses in open chest anesthetized dogs. A similar response was obtained in alpha-adrenergic blocked animals, but not with beta-adrenergic blocked or reserpine treated animals. All doses of pralidoxime significantly increased mean arterial pressure in control, beta-adrenergic blocked, and alpha-adrenergic blocked animals. Pralidoxime at 20 and 40 mg/kg also increased arterial pressure in reserpine treated animals. Heart rate was decreased in all but the alpha-adrenergic blocked animals with pralidoxime. The total peripheral resistance of the beta-blocked animals increased with every subsequent dose of pralidoxime although no significant increase was observed in controls. A smaller increase in total peripheral resistance was observed in reserpine-treated and alpha-adrenergic blocked animals. Significant increases in stroke volume and changes in stroke work were noted with all animals, each occurring at different atrial pressures depending on the treatment. The results suggest that pralidoxime directly stimulates the heart and vascular smooth muscle. Pralidoxime in dogs at high dosages, causes signs associated with its own anticholinesterase activity. Clinical signs of toxicity in dogs may be exhibited as muscle weakness, ataxia, vomiting, hyperventilation, seizures, respiratory arrest, and death. Protein Binding No binding to plasma proteins Interactions The pharmacokinetics of 5 mg/kg IV pralidoxime chloride (Protopam; I) when administered one hr after continuous infusion of thiamine hydrochloride (II) are described in 6 males. Subjects were given I alone and while receiving an infusion of II. After the addition of II, the urinary excretion of oxime was the same but the amount excreted in the first 3 hr was smaller; the plasma half-life of oxime lengthened; the plasma concentrations of oxime rose; and the intercompartmental clearances and rate constant for elimination for oxime fell. It was concluded that II and oxime compete for a common renal secretory mechanism or that II alters the membrane transport of oxime. BACKGROUND AND PURPOSE: Treatment of organophosphate poisoning with pralidoxime needs to be improved. Here we have studied the pharmacokinetics of pralidoxime after its intramuscular injection alone or in combination with avizafone and atropine using an auto-injector device. EXPERIMENTAL APPROACH: The study was conducted in an open, randomized, single-dose, two-way, cross-over design. At each period, each subject received either intramuscular injections of pralidoxime (700 mg), or two injections of the combination: pralidoxime (350 mg), atropine (2 mg), avizafone (20 mg). Pralidoxime concentrations were quantified using a validated LC/MS-MS method. Two approaches were used to analyse these data: (i) a non-compartmental approach; and (ii) a compartmental modelling approach. KEY RESULTS: The injection of pralidoxime combination with atropine and avizafone provided a higher pralidoxime maximal concentration than that obtained after the injection of pralidoxime alone (out of bioequivalence range), while pralidoxime AUC values were equivalent. Pralidoxime concentrations reached their maximal value earlier after the injection of the combination. According to Akaike and to goodness of fit criteria, the best model describing the pharmacokinetics of pralidoxime was a two-compartment with a zero-order absorption model. When avizafone and atropine were injected with pralidoxime, the best model describing pralidoxime pharmacokinetics becomes a two-compartment with a first-order absorption model. CONCLUSIONS AND IMPLICATIONS: The two approaches, non-compartmental and compartmental, showed that the administration of avizafone and atropine with pralidoxime results in a faster absorption into the general circulation and higher maximal concentrations, compared with the administration of pralidoxime alone. We have recently shown that the pyridinium aldoximes, best-known as therapeutic antidotes for chemical warfare nerve-agents, could markedly detoxify the carcinogenic tetrachloro-1,4-benzoquinone (TCBQ) via an unusual double Beckmann fragmentation mechanism. However, it is still not clear why pralidoxime (2-PAM) cannot provide full protection against TCBQ-induced biological damages even when 2-PAM was in excess. Here we show, unexpectedly, that TCBQ can also activate pralidoxime to generate a reactive iminyl radical intermediate in two-consecutive steps, which was detected and unequivocally characterized by the complementary application of ESR spin-trapping, HPLC/MS and nitrogen-15 isotope-labeling studies. The same iminyl radical was observed when TCBQ was substituted by other halogenated quinones. The end product of iminyl radical was isolated and identified as its corresponding reactive and toxic aldehyde. Based on these data, we proposed that the reaction of 2-PAM and TCBQ might be through the following two competing pathways: a nucleophilic attack of 2-PAM on TCBQ forms an unstable transient intermediate, which can decompose not only heterolytically to form 2-CMP via double Beckmann fragmentation, but also homolytically leading to the formation of a reactive iminyl radical in double-steps, which then via H abstraction and further hydrolyzation to form its corresponding more toxic aldehyde. Analogous radical homolysis mechanism was observed with other halogenated quinones and pyridinium aldoximes. This study represents the first detection and identification of reactive iminyl radical intermediates produced under normal physiological conditions, which provides direct experimental evidence to explain only the partial protection by 2-PAM against TCBQ-induced biological damages, and also the potential side-toxic effects induced by 2-PAM and other pyridinium aldoxime nerve-agent antidotes. When atropine and pralidoxime chloride are used together, the signs of atropinization (flushing, mydriasis, tachycardia, dryness of the mouth and nose) may occur earlier than might be expected when atropine is used alone. This is especially true if the total dose of atropine has been large and the administration of pralidoxime chloride has been delayed. The following precautions should be kept in mind in the treatment of anticholinesterase poisoning, although they do not bear directly on the use of pralidoxime chloride: since barbiturates are potentiated by the anticholinesterases, they should be used cautiously in the treatment of convulsions; morphine, theophylline, aminophylline, reserpine, and phenothiazine-type tranquilizers should be avoided in patients with organophosphate poisoning. Prolonged paralysis has been reported in patients when succinylcholine is given with drugs having anticholinesterase activity; therefore, it should be used with caution. Non-Human Toxicity Values LD50 Dog oral 190 mg/kg |
| 参考文献 | |
| 其他信息 |
Pralidoxime is a pyridinium ion that is 1-methylpyridinium substituted by a (hydroxyimino)methyl group at position 2. It has a role as a cholinergic drug, a cholinesterase reactivator, an antidote to organophosphate poisoning and an antidote to sarin poisoning.
Pralidoxime is an antidote to organophosphate pesticides and chemicals. Organophosphates bind to the esteratic site of acetylcholinesterase, which results initially in reversible inactivation of the enzyme. If given within 24 hours,after organophosphate exposure, pralidoxime reactivates the enzyme cholinesterase by cleaving the phosphate-ester bond formed between the organophosphate and acetylcholinesterase. Pralidoxime is a Cholinesterase Reactivator. The mechanism of action of pralidoxime is as a Cholinesterase Reactivator. See also: Pralidoxime Chloride (has salt form); Pralidoxime methyl sulfate (is active moiety of). Drug Indication For the treatment of poisoning due to those pesticides and chemicals of the organophosphate class which have anticholinesterase activity and in the control of overdosage by anticholinesterase drugs used in the treatment of myasthenia gravis. FDA Label Mechanism of Action Pralidoxime is an antidote to organophosphate pesticides and chemicals. Organophosphates bind to the esteratic site of acetylcholinesterase, which results initially in reversible inactivation of the enzyme. Acetylcholinesterase inhibition causes acetylcholine to accumulate in synapses, producing continuous stimulation of cholinergic fibers throughout the nervous systems. If given within 24 hours after organophosphate exposure, pralidoxime reactivates the acetylcholinesterase by cleaving the phosphate-ester bond formed between the organophosphate and acetylcholinesterase. Other reported pharmacologic effects of pralidoxime include depolarization at the neuromuscular junction, anticholinergic action, mild inhibition of cholinesterase, sympathomimetic effects, potentiation of the depressor action of acetylcholine in nonatropinized animals, and potentiation of the pressor action of acetylcholine in atropinized animals. However, the contribution of these effects to the therapeutic action of the drug has not been established. The principal pharmacologic effect of pralidoxime is reactivation of cholinesterase which has been recently inactivated by phosphorylation as the result of exposure to certain organophosphates. Pralidoxime removes the phosphoryl group from the active site of the inhibited enzyme by nucleophilic attack, regenerating active cholinesterase and forming an oxime complex. Pralidoxime also detoxifies certain organophosphates by direct chemical reaction and probably also reacts directly with cholinesterase to protect it from inhibition. Pralidoxime must be administered before aging of the inhibited enzyme occurs; after aging is completed, phosphorylated cholinesterase cannot be reactivated, and newly synthesized cholinesterase must replace the inhibited enzyme. Pralidoxime is not equally antagonistic to all anticholinesterases, partly because the time period required for aging of the inhibited enzyme varies and depends on the specific organophosphate bound to the cholinesterase. Therapeutic Uses Antidotes; Cholinesterase Reactivators /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Pralidoxime is included in the database. Protopam chloride is indicated as an antidote: 1. In the treatment of poisoning due to those pesticides and chemicals (e.g., nerve agents) of the organophosphate class which have anticholinesterase activity and 2. In the control of overdosage by anticholinesterase drugs used in the treatment of myasthenia gravis. The principal indications for the use of Protopam chloride are muscle weakness and respiratory depression. In severe poisoning, respiratory depression may be due to muscle weakness. /Included in US product label/ Pralidoxime chloride is used concomitantly with atropine for the treatment of nerve agent poisoning in the context of chemical warfare or terrorism. Pralidoxime chloride must be administered within minutes to hours following exposure to nerve agents to be effective. /Included in US product label/ For more Therapeutic Uses (Complete) data for 2-PAM (8 total), please visit the HSDB record page. Drug Warnings IM administration of pralidoxime may produce mild pain at the injection site. Rapid IV injection of pralidoxime has produced tachycardia, laryngospasm, muscle rigidity, and transient neuromuscular blockade; therefore, the drug should be administered slowly, preferably by IV infusion. IV administration of pralidoxime reportedly may also cause hypertension which is related to the dose and rate of infusion. Some clinicians recommend that the patient's blood pressure be monitored during pralidoxime therapy. For adults, IV administration of 5 mg of phentolamine mesylate reportedly quickly reverses pralidoxime-induced hypertension. Although pralidoxime is generally well-tolerated, dizziness, blurred vision, diplopia and impaired accommodation, headache, drowsiness, nausea, tachycardia, hyperventilation, maculopapular rash, and muscular weakness have been reported following administration of the drug. However, it is difficult to differentiate the toxic effects produced by atropine or organophosphates from those of pralidoxime, and the condition of patients suffering from organophosphate intoxication will generally mask minor signs and symptoms reported in normal subjects who receive pralidoxime. When atropine and pralidoxime are used concomitantly, signs of atropinism may occur earlier than when atropine is used alone, especially if the total dose of atropine is large and administration of pralidoxime is delayed. Excitement, confusion, manic behavior, and muscle rigidity have been reported following recovery of consciousness, but these symptoms have also occurred in patients who were not treated with pralidoxime. The following precautions should be kept in mind in the treatment of anticholinesterase poisoning, although they do not bear directly on the use of pralidoxime chloride: since barbiturates are potentiated by the anticholinesterases, they should be used cautiously in the treatment of convulsions; morphine, theophylline, aminophylline, reserpine, and phenothiazine-type tranquilizers should be avoided in patients with organophosphate poisoning. Prolonged paralysis has been reported in patients when succinylcholine is given with drugs having anticholinesterase activity; therefore, it should be used with caution. For more Drug Warnings (Complete) data for 2-PAM (11 total), please visit the HSDB record page. Pharmacodynamics Pralidoxime is to reactivate cholinesterase (mainly outside of the central nervous system) which has been inactivated by phosphorylation due to an organophosphate pesticide or related compound. The destruction of accumulated acetylcholine can then proceed, and neuromuscular junctions will again function normally. Pralidoxime also slows the process of "aging" of phosphorylated cholinesterase to a nonreactivatable form, and detoxifies certain organophosphates by direct chemical reaction. The drug has its most critical effect in relieving paralysis of the muscles of respiration. Because pralidoxime is less effective in relieving depression of the respiratory center, atropine is always required concomitantly to block the effect of accumulated acetylcholine at this site. Pralidoxime relieves muscarinic signs and symptoms, salivation, bronchospasm, etc., but this action is relatively unimportant since atropine is adequate for this purpose. |
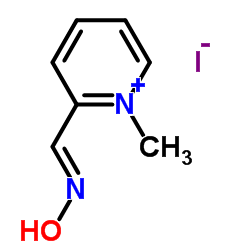
| 分子式 |
C7H9IN2O
|
|---|---|
| 分子量 |
264.07
|
| 精确质量 |
263.975
|
| CAS号 |
94-63-3
|
| 相关CAS号 |
Pralidoxime;6735-59-7
|
| PubChem CID |
135398747
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 密度 |
1.7439 g/ml
|
| 熔点 |
220 °C (dec.)(lit.)
|
| tPSA |
36.47
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
10
|
| 分子复杂度/Complexity |
125
|
| 定义原子立体中心数目 |
0
|
| SMILES |
C[N+]1=CC=CC=C1/C=N/O.[I-]
|
| InChi Key |
QNBVYCDYFJUNLO-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C7H8N2O.HI/c1-9-5-3-2-4-7(9)6-8-10;/h2-6H,1H3;1H
|
| 化学名 |
(NE)-N-[(1-methylpyridin-1-ium-2-yl)methylidene]hydroxylamine;iodide
|
| 别名 |
NSC 7760 NSC-7760 Pralidoxime iodide
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~250 mg/mL (~946.75 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7869 mL | 18.9344 mL | 37.8687 mL | |
| 5 mM | 0.7574 mL | 3.7869 mL | 7.5737 mL | |
| 10 mM | 0.3787 mL | 1.8934 mL | 3.7869 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。