| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
5-HT2A Receptor 4 nM (Ki) 5-HT6 Receptor Human 5-HT7 Receptor mAChR1 9.5 nM (Ki) mAChR4 11 nM (EC50) α2-adrenergic receptor 51 nM (Ki) D2 Receptor 75 nM (Ki)
|
|---|---|
| 体外研究 (In Vitro) |
氯氮平是一种非典型抗精神病药物,对几种血清素受体具有高亲和力。这种药物会导致5-羟色胺(2A)(5-HT)(2A)受体的反常下调,但其对其他血清素受体的调节尚未得到研究。我们研究了氯氮平和其他几种药物对转染HeLa细胞中单独表达的大鼠5-HT(6)和5-HT(7)受体的调节作用。5-HT(6)和5-HT(7)受体密度(B(max))均被激动剂5-甲酰胺色氨酸和反向激动剂甲氧苄啶降低。氯氮平降低5-HT(6)B(最大值)。这表明5-HT(6)受体也矛盾地被拮抗剂氯氮平下调。另一方面,氯氮平可增加5-羟色胺(7)受体B(max)。氯氮平对5-HT(6)和5-HT(7)受体水平的调节可能对这种非典型抗精神病药物的作用很重要[2]。
在中国仓鼠卵巢细胞中表达的人毒蕈碱M1-M5受体的功能测定中研究了氯氮平。氯氮平是毒蕈碱M4受体的完全激动剂(EC50=11nM),可抑制毛喉素刺激的cAMP积累。相比之下,氯氮平能有效拮抗激动剂诱导的其他四种毒蕈碱受体亚型的反应。选择性刺激M4受体可能部分解释氯氮平临床上观察到的高唾液分泌。此外,氯氮平独特的总体毒蕈碱特征可能有助于其非典型抗精神病疗效[4]。 |
| 体内研究 (In Vivo) |
盐酸氯氮平(25 mg/kg/天;腹膜内注射;21天)在麦角酸二乙酰胺诱发的精神病小鼠模型中表现出抗精神病作用[3]。
目的:本研究评估氯氮平慢性治疗对致幻血清素5-HT(2A)受体激动剂麦角酸二乙胺(LSD)作为精神病小鼠模型诱导的细胞和行为反应的影响。 方法:用25mg/kg/天的氯氮平对小鼠进行慢性治疗(21天)。实验在最后一次氯氮平给药后1、7、14和21天进行。[(3)H]测定小鼠体感皮层中氯胺酮结合和5-HT(2A)mRNA的表达。对所有5-HT(2A)激动剂诱导的头部抽搐行为、c-fos的表达以及LSD样特异性egr-1和egr-2的表达进行了检测。 结果:慢性氯氮平后1、7和14天,头部抽搐反应降低,[(3)H]酮丝氨酸结合下调。慢性氯氮平后1天,5-HT(2A)mRNA减少。慢性氯氮平治疗7天后,c-fos的诱导被挽救,但egr-1和egr-2没有。用氯氮平或慢性氟哌啶醇(1mg/kg/天)短期治疗(2天)后未观察到这些影响。 结论:我们的研究结果提供了一种慢性非典型抗精神病药物作用的小鼠模型,并表明5-HT(2A)受体的下调是这些持续治疗样作用的潜在机制[3]。 |
| 酶活实验 |
使用Prism 2.0通过非参数曲线拟合分析放射性配体结合。在5-HT6和5-HT7病例中,数据均与单部位模型吻合良好。计算每个试验中每种药物治疗条件的KD和Bmax值。所有药物治疗均未改变明显的KD;因此,Bmax值是使用2.4或6.3 nM的约束KD值确定的,这是分别与[3H]-5-HT(在表达5-HT7的细胞中)或[3H]-LSD(在表示5-HT6的细胞中。使用两到八种单独的结合试验来计算每种处理条件的Bmax值。Bmax值变化的统计分析是使用每种药物与赋形剂测定的Student双尾t检验完成的。为了数据呈现的清晰性,所有Bmax值均表示为对照±SEM的百分比[2]。
|
| 细胞实验 |
为了测试氯氮平从膜匀浆中洗出的效率,将氯氮平(1μM)或载体加入由2×TME中未经处理的细胞制备的膜中,并在室温下平衡1小时。然后将膜分成等份,在37°C下孵育15、30或60分钟,然后在20℃下造粒 000将膜重新悬浮在新鲜的2×TME中,如上所述在单一浓度下测量特异性结合(5-HT6为5 nM[3H]-LSD,5-HT7为1.67 nM[3H]-5-HT)[2]。
|
| 动物实验 |
Animal/Disease Models: Male 129 S6/Sv mice, lysergic acid diethylamide (LSD)-induced psychosis model[3]
Doses: 25 mg/kg/day Route of Administration: Intraperitoneal injection, 21 days Experimental Results: Decreased head-twitch response, reduced 5-HT2A mRNA, rescued induction of c-fos, but not egr-1 and egr-2. Materials and drug administration [3] Clozapine, methysergide, haloperidol, Lysergic acid diethylamide (LSD) were used. The injected doses (i.p.), calculated as salts, were clozapine, 1.5, 10.0, or 25.0 mg/kg; haloperidol, 1.0 mg/kg; and LSD, 0.24 mg/kg. These doses were selected based on previous findings with clozapine, haloperidol, and LSD in rodent models (Kuoppamaki et al. 1993; Kontkanen et al. 2002a, b; Gonzalez-Maeso et al. 2008; Gray et al. 2009). These doses represent approximately 12.5, 3.3, and 0.03 % of the LD50 of clozapine (200 mg/kg), haloperidol (30 mg/kg), and LSD (800 mg/kg) after i.p. administration in mice, respectively. Clozapine was injected after suspension in a minimal amount of DMSO supplemented with acetic acid and made up to volume with normal saline. Haloperidol was dissolved in saline. LSD was dissolved in a minimal amount of DMSO and made up to volume with normal saline. Control animals received vehicle injections. Drugs were administered in an injection volume of 100 μl/10 g mouse body weight. All other chemicals were obtained from standard sources. For effect of chronic treatment with clozapine on head-twitch behavior, chronic (21 days) treatment with clozapine started 3, 4, 5, and 6 weeks before the mice were injected with a single dose of LSD (i.e., 1, 7, 14, or 21 days after the last injection of chronic clozapine). Control mice were chronically (21 days) treated with vehicle and injected on the day of the experiment with a single dose of LSD (i.e., 1 day after the last injection of chronic vehicle). Injection of LSD was performed on the same day in all the groups of mice (i.e., acute LSD 1, 7, 14, or 21 after the last injection of chronic clozapine, and of acute LSD 1 day after the last injection of chronic vehicle). For the effect of chronic treatment with clozapine on the expression of 5-HT2A receptor (i.e., radioligand binding and mRNA), chronic (21 days) treatment with clozapine started 3, 4, 5, and 6 weeks before the mice were sacrificed (i.e., 1, 7, 14, or 21 days after the last injection of chronic clozapine). Control mice were chronically (21 days) treated with vehicle and sacrificed 1 day after the last injection of chronic vehicle. Mice were sacrificed on the same day in all the groups of mice (i.e., chronic clozapine 1, 7, 14, or 21 days after the last injection and chronic vehicle 1 day after the last injection). For the effect of chronic treatment with clozapine on the LSD-dependent induction of c-fos, egr-1, and egr-2 in mouse somatosensory cortex, treatment with clozapine or vehicle started 3, 4, 5, and 6 weeks before the mice were injected with a single dose of LSD or vehicle on the day of the experiment (i.e., 1, 7, 14, or 21 days after the last injection of chronic clozapine). Mice were sacrificed 60 min after injection with LSD. Similar conditions were used for chronic treatment with haloperidol. For short-term treatments, mice received one injection of clozapine, or vehicle, per day for 2 days, and experiments were performed 1 day after the last injection. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
In humans, clozapine tablets (25 mg and 100 mg) are equally bioavailable relative to a CLOZARIL solution. Following oral administration of clozapine 100 mg twice daily, the average steady-state peak plasma concentration was 319 ng/mL (range: 102 to 771 ng/mL), occurring at the average of 2.5 hours (range: 1 to 6 hours) after dosing. The average minimum concentration at steady state was 122 ng/mL (range: 41 to 343 ng/mL), after 100 mg twice daily dosing. Approximately 50% of the administered dose is excreted in the urine and 30% in the feces. The median volume of distribution of clozapine was calculated to be 508 L (272–1290 L). The median clearance of clozapine is calculated to be 30.3 L/h (14.4–45.2 L/h). Clozapine is almost completely metabolized prior to excretion and only trace amounts of unchanged drug are detected in the urine and feces. Approximately 50% of the administered dose is excreted in the urine and 30% in the feces. The demethylated, hydroxylated and N-oxide derivatives are components in both urine and feces. Pharmacological testing has shown the desmethyl metabolite to have only limited activity, while the hydroxylated and N-oxide derivatives were inactive. In man, clozapine tablets (25 mg and 100 mg) are equally bioavailable relative to a clozapine solution. Following a dosage of 100 mg b.i.d., the average steady-state peak plasma concentration was 319 ng/mL (range: 102 to 771 ng/mL), occurring at the average of 2.5 hours (range: 1 to 6 hours) after dosing. The average minimum concentration at steady-state was 122 ng/mL (range: 41 to 343 ng/mL), after 100 mg b.i.d. dosing. Food does not appear to affect the systemic bioavailability of clozapine. Thus, clozapine may be administered with or without food. Clozapine is approximately 97% bound to serum proteins. Clozapine is rapidly absorbed after both single and repeated oral doses, with steady-state concentrations attained within eight to ten days after beginning therapy. Metabolism / Metabolites Clozapine is almost completely metabolized prior to excretion, and only trace amounts of unchanged drug are detected in the urine and feces. Clozapine is a substrate for many cytochrome P450 isozymes, in particular CYP1A2, CYP2D6, and CYP3A4.The unmethylated, hydroxylated, and N-oxide derivatives are components in both urine and feces. Pharmacological testing has shown the desmethyl metabolite (norclozapine) to have only limited activity, while the hydroxylated and N-oxide derivatives were inactive. Manic and schizophrenic patients were given neuroleptic clozapine at 300-500 mg daily and metabolites of clozapine were isolated from urine and analyzed by gas chromatography-mass spectrometry. Clozapine was converted into 2 metabolites by replacement of chlorine atom by a hydroxyl or methylsulfide group. Further metabolites were the N-demethyl deriv of 1st two metabolites. A metabolite with an oxidized piperazine ring was also found, and possibility of a metabolite with an oxidized sulfur is suggested. /Clozapine/ is metabolized to N-oxideclozapine and N-desmethylclozapine, which have less pharmacological activity than the parent compound and are excreted in the urine and, to a lesser extent, in the feces. Clozapine has known human metabolites that include Clozapine N-glucuronide, Clozapine-N-oxide, and N-Desmethylclozapine. Biological Half-Life The mean elimination half-life of clozapine after a single 75 mg dose was 8 hours (range: 4 to 12 hours), compared to a mean elimination half-life of 12 hours (range: 4 to 66 hours), after achieving a steady state with 100 mg twice daily dosing. A comparison of single-dose and multiple-dose administration of clozapine demonstrated that the elimination half-life increased significantly after multiple dosing relative to that after single-dose administration, suggesting the possibility of concentration-dependent pharmacokinetics. The mean elimination half-life of clozapine after a single 75 mg dose was 8 hours (range: 4 to 12 hours), compared to a mean elimination half-life, after achieving steady-state with 100 mg b.i.d. dosing, of 12 hours (range: 4 to 66 hours). |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Clozapine has been shown to be an effective, relatively rapid-acting, broad-spectrum antipsychotic agent in both uncontrolled and controlled studies of patients with schizophrenia.Clozapine has been used in a limited number of patients with advanced, idiopathic parkinsonian syndrome for the management of dopaminomimetic psychosis associated with antiparkinsonian drug therapy, but adverse effects such as sedation, confusion, and increased parkinsonian manifestations may limit the benefit of clozapine therapy in these patients. Clozapine is used to reduce the risk of recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder who are judged to be at chronic risk for such behavior, based on history and recent clinical state. Although the safety and efficacy of clozapine in children and adolescents younger than 16 years of age have not been established, the drug has been successfully used for the management of childhood-onset schizophrenia in a limited number of treatment-resistant children and adolescents. Clozapine is used for the symptomatic management of schizophrenia in severely ill patients whose disease fails to respond adequately to other antipsychotic therapy. HUMAN EXPOSURE AND TOXICITY: The most frequent adverse effects of clozapine involve the central and autonomic nervous systems (e.g., drowsiness or sedation, hypersalivation) and the cardiovascular system (e.g., tachycardia, hypotension). While the frequency and severity of some adverse effects (e.g., extrapyramidal reactions, tardive dyskinesia) appear to be less with clozapine than with other antipsychotic agents, other potentially serious adverse effects (e.g., agranulocytosis, seizures) may occur more frequently with clozapine therapy, and the potential risks and benefits should be evaluated carefully whenever therapy with the drug is considered. Although it has been suggested that a local genetic or environmental factor or factors may have been involved in the Finnish cases, the existence of such a factor has not been documented. During a 2 month period in Finland there were 18 reports of severe blood disorders (9 fatal) associated with clozapine. Agranulocytosis accounted for 8 of the deaths and leukemia probably for the ninth. Experience in 22 other countries outside Finland where clozapine had been marketed indicated an incidence of agranulocytosis of 0.3 per 1000 compared with an incidence almost 20 times as high in Finland and with 0.1 to 0.8 per 1000 for other tricyclic neuroleptics. patients who received flexible dosages of clozapine (mean dosage: 274.2 mg daily) for approximately 2 years had a 26% reduction in their risk for suicide attempts or hospitalization to prevent suicide compared with those who received flexible dosages of olanzapine (mean dosage: 16.6 mg daily); the treatment-resistant status of patients was not predictive of response to clozapine or olanzapine. ANIMAL STUDIES: Repeated oral administration to rats (6 months) and to dogs (3 months) decreased wt gain with doses of 20 mg/kg or more in rats and of 10 mg/kg or more in dogs. Hepatic hypertrophy, which was not strictly dose-dependent, was not associated with either histological changes or changes in blood chemistry and was completely reversible after discontinuation of treatment. No toxic signs were observed in rats or in dogs. Clozapine in daily oral doses of 20 or 40 mg/kg to rats and rabbits had no teratogenic effects and no influence on the fertility of male and female rats. At 40 mg/kg, however, clozapine inhibited growth of suckling young of treated mothers. Fertility of F1 treated mothers was normal and development of F2 showed no abnormalities. Clozapine hydrochloride inhibited conditioned avoidance behavior in rats, also inhibited writhing syndrome induced by phenylbenzoquinone in mice, and decreased body temp. Clozapine hydrochloride antagonized tremor and lacrimation induced by oxotremorine in mice, decreased the acute toxicity of physostigmine and 5-hydroxyindol acetate level in brain. Non-Human Toxicity Values LD50 Rat iv 41.6 mg/kg LD50 Rat sc 240 mg/kg LD50 Rat im 210 mg/kg LD50 Rat oral 251 mg/kg |
| 参考文献 |
|
| 其他信息 |
Ever since clozapine was first synthesized and tested, it showed the unique property of having antipsychotic action but no Parkinson-like motor side effects. The antipsychotic basis of clozapine is to transiently occupy dopamine D2 receptors in the human striatum, in contrast to haloperidol and chlorpromazine, which have a prolonged occupation of D2 receptors. The chemical structure of clozapine facilitates a relatively rapid dissociation from D2 receptors. After short-term occupation of D2 receptors, peak neural activity raises synaptic dopamine, which then displaces clozapine. While clozapine also occupies other types of receptors, they may not have a significant role in preventing parkinsonism. Clozapine's transient occupation of D2 receptors permits patients to move easily and comfortably. [1]
Rationale: In schizophrenia patients, optimal treatment with antipsychotics requires weeks to months of sustained drug therapy. However, single administration of antipsychotic drugs can reverse schizophrenia-like behavioral alterations in rodent models of psychosis. This raises questions about the physiological relevance of such antipsychotic-like activity. Objective: This study evaluates the effects of chronic treatment with clozapine on the cellular and behavioral responses induced by the hallucinogenic serotonin 5-HT(2A) receptor agonist lysergic acid diethylamide (LSD) as a mouse model of psychosis. Method: Mice were treated chronically (21 days) with 25 mg/kg/day clozapine. Experiments were conducted 1, 7, 14, and 21 days after the last clozapine administration. [(3)H]Ketanserin binding and 5-HT ( 2A ) mRNA expression were determined in mouse somatosensory cortex. Head-twitch behavior, expression of c-fos, which is induced by all 5-HT(2A) agonists, and expression of egr-1 and egr-2, which are LSD-like specific, were assayed. Results: Head-twitch response was decreased and [(3)H]ketanserin binding was downregulated in 1, 7, and 14 days after chronic clozapine. 5-HT ( 2A ) mRNA was reduced 1 day after chronic clozapine. Induction of c-fos, but not egr-1 and egr-2, was rescued 7 days after chronic clozapine. These effects were not observed after short treatment (2 days) with clozapine or chronic haloperidol (1 mg/kg/day). Conclusion: Our findings provide a murine model of chronic atypical antipsychotic drug action and suggest downregulation of the 5-HT(2A) receptor as a potential mechanism involved in these persistent therapeutic-like effects.[3] |
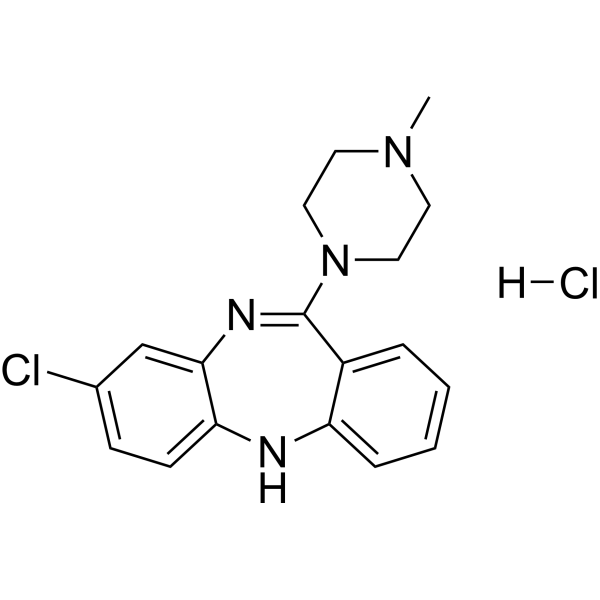
| 分子式 |
C18H20CL2N4
|
|---|---|
| 分子量 |
363.28
|
| 元素分析 |
C, 59.51; H, 5.55; Cl, 19.52; N, 15.42
|
| CAS号 |
54241-01-9
|
| 相关CAS号 |
54241-01-9 (HCl); 34233-69-7 (N-oxide); 5786-21-0
|
| PubChem CID |
135524906
|
| 外观&性状 |
Typically exists as solids at room temperature
|
| 沸点 |
477.8ºC at 760 mmHg
|
| 闪点 |
242.8ºC
|
| LogP |
2.281
|
| tPSA |
30.87
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
446
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CN1CCN(CC1)C2=NC3=C(C=CC(=C3)Cl)NC4=CC=CC=C42.Cl
|
| 别名 |
HF 1854 hydrochloride; Clozapine hydrochloride; 54241-01-9; Clozapine (hydrochloride); CLZ-ChemoNM; 80862U56A3; 3-chloro-6-(4-methylpiperazin-1-yl)-11H-benzo[b][1,4]benzodiazepine;hydrochloride; CHEMBL538973; Clozapine HCl;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7527 mL | 13.7635 mL | 27.5270 mL | |
| 5 mM | 0.5505 mL | 2.7527 mL | 5.5054 mL | |
| 10 mM | 0.2753 mL | 1.3763 mL | 2.7527 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Clozapine for the Prevention of Violence in Schizophrenia: a Randomized Clinical Trial
CTID: NCT05208190
Phase: Phase 4 Status: Recruiting
Date: 2024-04-02