| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |

| 靶点 |
Negative control (NC) for Zn(II) Protoporphyrin
|
|---|---|
| 参考文献 | |
| 其他信息 |
Heme oxygenase-1 (HO-1) catalyzes the oxidation of heme to biologically active products: carbon monoxide (CO), biliverdin, and ferrous iron. It participates in maintaining cellular homeostasis and plays an important protective role in the tissues by reducing oxidative injury, attenuating the inflammatory response, inhibiting cell apoptosis, and regulating cell proliferation. HO-1 is also an important proangiogenic mediator. Most studies have focused on the role of HO-1 in cardiovascular diseases, in which its significant, beneficial activity is well recognized. A growing body of evidence indicates, however, that HO-1 activation may play a role in carcinogenesis and can potently influence the growth and metastasis of tumors. HO-1 is very often upregulated in tumor tissues, and its expression is further increased in response to therapies. Although the exact effect can be tissue specific, HO-1 can be regarded as an enzyme facilitating tumor progression. Accordingly, inhibition of HO-1 can be suggested as a potential therapeutic approach sensitizing tumors to radiation, chemotherapy, or photodynamic therapy. [1]
Heme oxygenase (HO) has been shown to be important for attenuating the overall production of reactive oxygen species (ROS) through its ability to degrade heme and to produce carbon monoxide (CO), biliverdin/bilirubin, and the release of free iron. Excess free heme catalyzes the formation of ROS, which may lead to endothelial cell (EC) dysfunction as seen in numerous pathological conditions including hypertension and diabetes, as well as ischemia/reperfusion injury. The upregulation of HO-1 can be achieved through the use of pharmaceutical agents, such as metalloporphyrins and some HMG-CoA reductase inhibitors. Among other agents, atrial natriretic peptide and donors of nitric oxide (NO) are important modulators of the heme-HO system, either through induction of HO-1 or the biological activity of its products. Gene therapy and gene transfer, including site- and organ-specific targeted gene transfer, have become powerful tools for studying the potential role of HO-1/HO-2 in the treatment of various cardiovascular diseases as well as diabetes. HO-1 induction by pharmacological agents or gene transfer of human HO-1 into endothelial cells (ECs) in vitro increases cell-cycle progression and attenuates Ang II, TNF-, and heme-mediated DNA damage; administration in vivo acts to correct blood pressure elevation following Ang II exposure. Moreover, site-specific delivery of HO-1 to renal structures in spontaneously hypertensive rats (SHR), specifically to the medullary thick ascending limb of the loop of Henle (mTALH), has been shown to normalize blood pressure and provide protection to the mTAL against oxidative injury. In other cardiovascular situations, delivery of human HO-1 to hyperglycemic rats significantly lowers superoxide (O(2)(-)) levels and prevents EC damage and sloughing of vascular EC into the circulation. In addition, administration of human HO-1 to rats in advance of ischemia/reperfusion injury considerably reduces tissue damage. The ability to upregulate HO-1 through pharmacological means or through the use of gene therapy may offer therapeutic strategies for cardiovascular disease in the future. This review discusses the implications of HO-1 delivery during the early stages of cardiovascular system injury or in early vascular pathology and suggests that pharmacological agents that regulate HO activity or HO-1 gene delivery itself may become powerful tools for preventing the onset or progression of certain cardiovascular pathologies. [2] Adiponectin, an abundant adipocyte-derived plasma protein that modulates vascular function in type 2 diabetes, has been shown to provide cytoprotection to both pancreatic and vascular systems in diabetes. Therefore, we examined whether up-regulation of heme oxygenase (HO)-1 ameliorates the levels of inflammatory cytokines and influences serum adiponectin in Zucker fat (ZF) rats. ZF rats displayed a decrease in both HO activity and HO-1 and HO-2 protein levels and an increase in tumor necrosis factor (TNF)-alpha and interleukin (IL)-6 compared with Zucker lean (ZL) rats. Treatment of ZF animals with 2 mg/kg cobalt protoporphyrin IX (CoPP) increased protein levels of HO-1 and HO activity, but HO-2 was unaffected. The increase in HO-1 was associated with a decrease in superoxide levels (p < 0.05) and an increase in plasma adiponectin (p < 0.005), compared with untreated ZF rats. CoPP treatment decreased visceral and s.c. fat content, and it reduced weight gain (p < 0.01). In addition, the inflammatory cytokines TNF-alpha and IL-6 were decreased (p < 0.04 and p < 0.008, respectively). Treatment of human bone marrow-derived adipocytes cultured with CoPP resulted in an increase in HO-1 and a decrease in superoxide levels. Up-regulation of HO-1 caused adipose remodeling, smaller adipocytes, and increased adiponectin secretion in the culture medium of human bone marrow-derived adipocytes. In summary, this study demonstrates that the antiobesity effect of HO-1 induction results in an increase in adiponectin secretion, in vivo and in vitro, a decrease in TNF-alpha and IL-6, and a reduction in weight gain. These findings highlight the pivotal role and symbiotic relationship of HO-1 and adiponectin in the modulation of the metabolic syndrome phenotype. [3] |
| 分子式 |
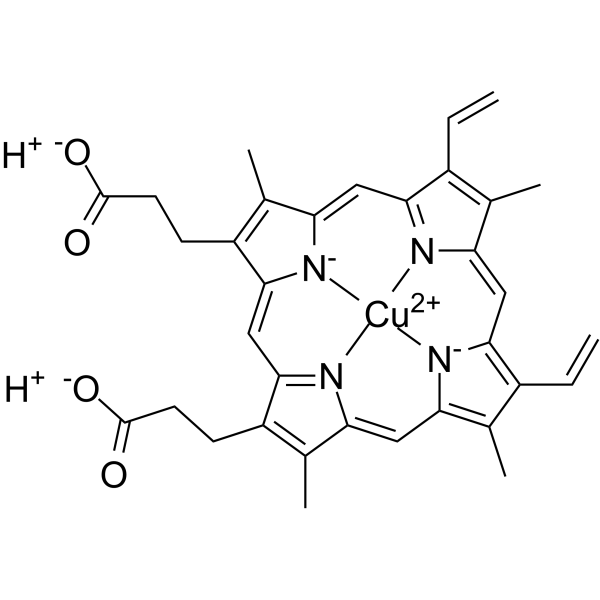
C34H32CUN4O4
|
|---|---|
| 分子量 |
624.19
|
| 精确质量 |
623.172
|
| 元素分析 |
C, 65.42; H, 5.17; Cu, 10.18; N, 8.98; O, 10.25
|
| CAS号 |
14494-37-2
|
| 相关CAS号 |
Mg(II) protoporphyrin IX;14947-11-6;Mn(II) protoporphyrin IX;21393-64-6;Ni(II) protoporphyrin IX;15415-30-2;Ga(III) protoporphyrin IX;222556-71-0;Cr(III) protoporphyrin IX;84640-43-7;Cd(II) protoporphyrin IX;80216-25-7;Pt(II) protoporphyrin IX;98303-94-7
|
| PubChem CID |
3500653
|
| 外观&性状 |
Brown to reddish brown solid powder
|
| LogP |
2.322
|
| tPSA |
94.32
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
43
|
| 分子复杂度/Complexity |
1580
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC1=C(C2=CC3=NC(=CC4=C(C(=C([N-]4)C=C5C(=C(C(=N5)C=C1[N-]2)C)C=C)C)C=C)C(=C3CCC(=O)O)C)CCC(=O)O.[Cu+2]
|
| InChi Key |
ASFPSNQTLAUXFI-UHFFFAOYSA-L
|
| InChi Code |
InChI=1S/C34H34N4O4.Cu/c1-7-21-17(3)25-13-26-19(5)23(9-11-33(39)40)31(37-26)16-32-24(10-12-34(41)42)20(6)28(38-32)15-30-22(8-2)18(4)27(36-30)14-29(21)35-25;/h7-8,13-16H,1-2,9-12H2,3-6H3,(H4,35,36,37,38,39,40,41,42);/q;+2/p-2
|
| 化学名 |
copper;3-[18-(2-carboxyethyl)-7,12-bis(ethenyl)-3,8,13,17-tetramethylporphyrin-21,23-diid-2-yl]propanoic acid
|
| 别名 |
cu(ii) protoporphyrin ix; 14494-37-2; cu(ii)protoporphyrinix; G91025; Cuprate(2-),[7,12-diethenyl-3,8,13,17-tetramethyl-21H,23H-porphine-2,18-dipropanoato(4-)-kN21,kN22,kN23,kN24]-, dihydrogen, (SP-4-2)-
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6021 mL | 8.0104 mL | 16.0208 mL | |
| 5 mM | 0.3204 mL | 1.6021 mL | 3.2042 mL | |
| 10 mM | 0.1602 mL | 0.8010 mL | 1.6021 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。