| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
NMDA Receptors
|
|---|---|
| 体外研究 (In Vitro) |
化合物MK-801[(+)-5-甲基-10,11-二氢-5H-二苯并[a,d]环庚烯-5,10-亚胺马来酸酯]是一种强效抗惊厥药,口服后具有活性,其作用机制尚不清楚。我们在大鼠脑膜中检测到[3H]MK-801的高亲和力(Kd=37.2+/-2.7 nM)结合位点。这些位点是热不稳定的、立体选择性的和区域特异性的,海马体的位点密度最高,其次是大脑皮层、纹状体和脑桥髓质。小脑中未检测到结合。MK-801结合位点表现出一种新的药理学特征,因为这些位点上没有一种主要的神经递质候选物是活性的。唯一能够竞争[3H]MK-801结合位点的化合物是已知能够阻断N-甲基-D-天冬氨酸(N-Me-D-Asp)受体亚型介导的兴奋性氨基酸反应的物质。这些药物包括游离麻醉剂苯环利定和氯胺酮以及西格玛型阿片类药物N-烯丙基甲氧基丙胺(SKF 10047)。使用大鼠皮质切片制备的体外神经生理学研究表明,MK-801对N-Me-D-Asp的去极化反应具有强效、选择性和非竞争性拮抗作用,但对红藻氨酸或奎司琼酸盐没有。苯环利定、氯胺酮、SKF 10047和MK-801作为N-Me-D-Asp拮抗剂的效力与其作为[3H]MK-801结合抑制剂的效力密切相关(r=0.99)。这表明MK-801结合位点与N-Me-D-Asp受体有关,并解释了MK-801作为抗惊厥药的作用机制[1]。
|
| 体内研究 (In Vivo) |
在动物模型中,马来酸地佐环平可用于创建精神分裂症模型。最近的研究表明,与药物有关的记忆在暴露于环境线索后会被重新激活,并可能经历重新巩固,这一过程可以增强记忆。相反,某些药物可能会破坏再巩固,从而削弱与药物相关的记忆。几项研究已经证明,使用药物诱导的条件性位置偏好(CPP)任务会破坏记忆的再巩固,但没有研究探讨在可卡因预充注射后,可卡因相关的记忆是否会在可卡因自我给药动物中受到类似的破坏,这会有力地恢复药物寻求行为。在这里,我们使用可卡因诱导的CPP和可卡因自我给药来研究在重新激活之前给予N-甲基-D-天冬氨酸受体拮抗剂(+)-5-甲基-10,11-二氢-5H-二苯并[a,D]环庚烯-5,10-马来酸亚胺(MK-801)是否会抑制随后可卡因引发的恢复(破坏再巩固)。在CPP背景下可卡因相关记忆重新激活之前,在大鼠体内全身注射MK-801(腹腔注射0.05或0.20mg/kg)会减弱随后可卡因引发的恢复,而在CPP环境中未接受重新激活的大鼠则不会出现中断。然而,在接受过自我给药可卡因训练的大鼠中,在两种不同类型的再激活过程之前全身给药MK-801对随后可卡因引发的杠杆按压行为的恢复没有影响。因此,MK-801的系统给药破坏了可卡因相关记忆对CPP的再巩固,但对自我给药没有影响。这些发现表明,可卡因CPP和自我给药不会使用类似的神经化学过程来破坏再巩固,或者自我给药大鼠的可卡因相关记忆不会经历再巩固,这是通过可卡因恢复条件下的杠杆按压行为来评估的[5]。
研究了单独吗啡(MOR:10和20mg/kg,皮下注射)、单独MK-801(地佐西平:0.03、0.1、0.3和1mg/kg,腹腔注射)以及MOR与MK-801的组合对小鼠行走的影响。MK-801在0.3和1mg/kg时,但在0.03和0.1mg/kg时没有显著增加小鼠的行走能力。尽管反复给药MK-801(0.3和1mg/kg)的小鼠在个体剂量的步行增加效应中分别表现出增强和减弱,但它们对MOR(10mg/kg)的挑战表现出明显高于生理盐水处理的小鼠的敏感性。MOR(10和20mg/kg)的重复给药诱导了步行增加效果的逐渐增强。反复给予MOR(10mg/kg)的小鼠对MK-801(0.03-0.3mg/kg)的敏感性显著增加。MOR与MK-801的联合用药增强了步行增加的效果,重复联合用药诱导了效果的逐渐增强,但MOR(10或20 mg/kg)与MK-802(1 mg/kg)的联合用药除外。然而,除了MOR(20mg/kg)与MK-801(1mg/kg)联合使用的情况外,任何剂量的MK-801都不会改变MOR致敏的诱导,MK-801具有高毒性(即引发死亡或垂死状态)。另一方面,同时用SCH 23390(0.05 mg/kg,皮下注射)或尼莫地平(0.05 mg/kg)治疗,或用利血平(1 mg/kg,皮下移植)预处理4小时,用α-甲基对酪氨酸(200 mg/kg,腹腔注射)预处理6小时,部分降低了MOR(10 mg/kg)和MK-801(0.3 mg/kg)的步行增加作用。纳洛酮(1mg/kg,皮下注射)同时治疗选择性地降低了MOR的效果。然而,同时用阿扑吗啡(0.1mg/kg,皮下注射)治疗并没有改变任何一种药物的效果。这些结果表明,MOR和MK-801的步行增加作用的特征彼此相似,MK-801重复治疗可诱导对MOR的交叉致敏,反之亦然[6]。 |
| 细胞实验 |
神经元从2至6天大的Long-Evans大鼠幼崽的视觉皮层中分离出来,并在培养基中生长5-43天,如所述(21)。在全细胞和外部膜片钳配置中测量了由氨基酸激发激活的电流。移液管中含有120甲基磺酸铯、5 CsCI、10 Cs2EGTA、5 Mg(OH)2、5 MgATP、1 Na2GTP和10 Hepes的内溶液(单位为mM)(用CsOH将pH值调节至7.4)。外部溶液(单位为mM)为160 NaCl、2 CaC12和10 Hepes(pH 7.40)。在全细胞实验中,将300 nM河豚毒素和10 kLM荷包牡丹碱甲基碘添加到外部溶液中以抑制自发活动。MK-801是Paul Anderson的礼物,是从2-50mM的乙醇储备溶液中加入的,储存在-20℃下。乙醇的最终浓度<0.1%。将细胞或贴片浸泡在对照或含激动剂的外部溶液中,该外部溶液由重力供给的7-10个微毛细管线性阵列中的一个流出。通过相对于细胞(整个细胞)移动试管阵列或相对于试管(贴片)移动移液管,可以快速更换溶液。所有实验均在20-250C下进行[3]。
|
| 动物实验 |
Systemic injection of Dizocilpine/MK-801 (0.05 or 0.20 mg/kg administered intraperitoneally) in rats just prior to reactivation of the cocaine-associated memory in the CPP context attenuated subsequent cocaine-primed reinstatement, while no disruption occurred in rats that did not receive reactivation in the CPP context. However, in rats trained to self-administer cocaine, systemic administration of MK-801 just prior to either of two different types of reactivation sessions had no effect on subsequent cocaine-primed reinstatement of lever-pressing behavior. Thus, systemic administration of MK-801 disrupted the reconsolidation of a cocaine-associated memory for CPP but not for self-administration. These findings suggest that cocaine-CPP and self-administration do not use similar neurochemical processes to disrupt reconsolidation or that cocaine-associated memories in self-administering rats do not undergo reconsolidation, as assessed by lever-pressing behavior under cocaine reinstatement conditions.[5]
Subjects [5] Male Sprague-Dawley and Long-Evans Hooded rats weighing 280–350 g at the start of the experiment were housed in a temperature- and humidity-controlled colony room with a 12-h light/dark cycle (lights on at 6:00 a.m.). Sprague-Dawley rats were used for all CPP studies, and our initial self-administration studies used Long-Evans rats because of their higher general activity levels and thus higher initial lever pressing during acquisition of the self-administration task. However, to ensure that there were no strain differences in the effects of Dizocilpine/MK-801 on self-administration behavior, we also used Sprague-Dawley rats to test the effects of the highest dose of MK-801 compared with Saline vehicle in this strain. No significant differences were found for the effects of MK-801, so the data from both strains were pooled. Animals undergoing self-administration were housed in a 12-h reverse light/dark cycle (lights on at 6:00 p.m.). Experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and experimental protocols were approved by the University Animal Care and Use Committee. Animals were housed two per cage for the CPP studies and individually for the self-administration studies. Food and water were provided ad libitum except for when animals were engaged in experiments. Drug administration [5] Dizocilpine(+)-MK-801 hydrogen maleate was dissolved in sterile saline for i.p. injection (1 mL/kg). The doses chosen were 0.05 and 0.20 mg/kg, based on previous work by Przybyslawski and Sara (1997). Surgery [5] Self-administration surgery was conducted according to a modification of McFarland and Kalivas (2001). Rats were anesthetized with zyket (ketamine 87 mg/kg + xylazine 13 mg/kg) given intramuscularly prior to implanting a chronic indwelling i.v. catheter. The catheter was surgically implanted into the right jugular vein, and the distal end was led subcutaneously to the back between the scapulas. Catheters were constructed from Silastic tubing (9 cm; inner diameter 0.025 in, outer diameter 0.047 in) connected to a back-mount cannula pedestal, a bent 22-gauge metal cannula encased within a plastic screw connector attached to a polyester mesh (Plastics One). A small ball of silicone sealant was placed ∼2.8 cm from the end of the catheter. The right jugular vein was isolated, the most anterior portion of the vein was tied shut, and a small incision was made. The distal end of the catheter was inserted into the vein until the silicone ball was flush with the vein. The vein was secured by tying suture thread on both sides of the silicone ball; additionally, the thread on both sides was tied together. Immediately after surgery, the catheter was injected with 0.1 mL of locking solution: heparin (500 U/mL), gentamicin (5 mg/mL), and glycerol (60%) in sterile saline. Incisions were sutured, and the animal was given 5–7 d to recover. After surgery, the catheter was flushed daily with 0.1 mL of heparin (10 U/mL) and gentamicin antibiotic (5 mg/mL) in sterile saline to help protect against infection and catheter occlusion. Behavioral procedures [5] CPP [5] All CPP studies were conducted during the same time of day. The proposed studies employed a three-compartment CPP apparatus as previously described (Brown et al. 2007). Briefly, the procedure consisted of a preconditioning preference test, training for 8 d (4 saline pairings alternating with 4 cocaine pairings), testing for CPP acquisition followed by extinction sessions, and cocaine-primed reinstatement with a 10 mg/kg, i.p. dose of cocaine (Brown et al. 2007). Except for the training days, rats had access to all three compartments of the CPP apparatus. In Experiment 1, we tested whether Dizocilpine/MK-801 would impair reconsolidation of the memory for the cocaine-associated context during reinstatement testing. Animals underwent preconditioning, conditioning, testing, and extinction as described above, and on Reactivation Day 1, rats received saline or MK-801 (0.05 mg/kg or 0.20 mg/kg, i.p.) 30 min prior to a cocaine injection (10 mg/kg, i.p.) and placed immediately into the central compartment of the CPP box (Reactivation Day 1). Rats were allowed to explore all three compartments. The next day, the procedure from Reactivation Day 1 was repeated (Reactivation Day 2). This procedure was given for 2 d because our previous studies using a different pharmacological agent (Brown et al. 2007) indicated that one day of memory reactivation was not sufficient to disrupt subsequent cocaine-primed reinstatement. The following day, animals were tested for cocaine-primed reinstatement without any prior injection of either saline or MK-801 before being placed into the CPP box (Reinstatement Day). Rats were allowed to explore all three compartments. Experiment 2 was identical to Experiment 1 with the exception of the cage location where Dizocilpine/MK-801 and cocaine injection took place on Reactivation Days 1 and 2. In Experiment 2, animals were given saline or MK-801 followed by cocaine 30 min later in the home cage instead of in the CPP apparatus for the two days of “reactivation.” This was done to determine whether reactivation of the memory for the cocaine-associated context by cocaine in the CPP context was necessary for the ability of MK-801 to disrupt reconsolidation. Animals underwent preconditioning, conditioning, testing, and extinction as described above but animals were injected with saline or MK-801 (0.20 mg/kg, i.p.) 30 min prior to a cocaine injection (10 mg/kg, i.p.) in the home cage. Animals remained in the home cages, and the next day, the procedure from the first day of reactivation was repeated. The following day, animals were tested for cocaine-primed reinstatement in their CPP box without any prior microinjection of saline or MK-801, exactly as described for the Reinstatement Day in Experiment 1 above. |
| 药代性质 (ADME/PK) |
Dizocilpine (MK-801) is a non-competitive NMDA receptor antagonist with high binding affinity, requiring an open channel for receptor blockade. Key pharmacokinetic characteristics include:
1. Bioavailability & Absorption o While specific bioavailability data for dizocilpine is not provided in the sources, its structural analog orphenadrine (an NMDA antagonist with similar properties) demonstrates blood-brain barrier penetration, suggesting dizocilpine may share this trait. 2. Metabolism & Elimination o Studies on reeler mice indicate dizocilpine’s efficacy correlates with GABAergic modulation, implying potential hepatic metabolism involving neurotransmitter pathways. o Comparative pharmacokinetic data from paliperidone derivatives suggest rapid metabolism may occur for certain CNS-targeting drugs, though dizocilpine’s exact metabolic profile remains unspecified. 3. Pharmacodynamic Interactions o Dizocilpine’s NMDA receptor blockade is enhanced in models of synaptic plasticity dysfunction, suggesting context-dependent pharmacokinetic-pharmacodynamic relationships. For precise quantification (e.g., Tmax, half-life), additional data beyond the current search results would be required. |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
... The purpose of the present study was to investigate the effect of dizocilpine maleate (MK-801), non-competitive NMDA glutamate receptor antagonist, on neurotoxic effect of the prolonged treatment with the high dose of dexamethasone (DEX). The results showed that DEX (120 mg/kg/day for 7 days) impaired the long-term memory and the motor coordination, reduced the body weight and induced the lethality of mice. The morphological and ultrastructural study have confirmed damage to hippocampal neurons especially in the CA3 region after the prolonged treatment with DEX alone. Damaged pyramidal neurons showed robust changes in the shape of the nucleus and cytoplasm condensation. MK-801 alone (at non-toxic dose of 0.3 mg/kg/day), changed neither the behavior of mice nor morphology of the hippocampal neurons. However, it did not prevent the neurotoxic effects of DEX. On the contrary, it intensified DEX-induced neurotoxicity. ...In /a/ preliminary study, methamphetamine (METH) at 2.5 mg/kg, but not at 1.0 mg/kg, induced a delayed increase in glutamate levels in the nucleus accumbens (NAc). /It was hypothesized/ that repeated increases in glutamate levels produces behavioral sensitization to a selective uncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist, dizocilpine (MK-801), and that an activation of protein kinase C (PKC) plays an important role for this sensitization. ... This study was conducted to confirm delayed increases in glutamate levels induced by a higher dose of METH (2.5 mg/kg), and to examine the effect of straurosporine, a PKC inhibitor, on the higher dose of METH-induced sensitization to dizocilpine. ... METH at 2.5 mg/kg, but not at 1.0 mg/kg, induced delayed increases in glutamate levels. The acute administration of staurosporine did not affect the locomotor activity by a single injection of METH (2.5 mg/kg). Repeated METH administrations (2.5 mg/kg, once in every other day, for five times) developed behavioral sensitization to the locomotion-inducing effect of dizocilpine (0.2 mg/kg), a selective uncompetitive NMDA receptor antagonist. Staurosporine (0.1 mg/kg), given 120 min later for every METH treatment, inhibited the development of behavioral sensitization to dizocilpine. ... These results suggest the involvement of increased glutamate levels and an activation of PKC in delayed-induced synaptic and cellular plasticity underlying the higher dose of METH-induced behavioral sensitization to dizocilpine. ... The present study was designed to investigate the importance of sex differences in the interaction between dizocilpine (MK-801) pretreatment and acute cold-restraint stress (CRS) in pentylenetetrazole (PTZ)-induced seizures in Swiss albino mice. ... A CRS protocol was applied to mice to investigate the interaction between MK-801 pretreatment (30 min before CRS) and stress (followed by PTZ injection) in epilepsy susceptibility. For this purpose, 6 groups were designated: (1) PTZ control group (received only PTZ); (2) stress group (received stress and PTZ); (3) saline group (received saline and PTZ); (4) MK-801 group (received MK-801 and PTZ); (5) saline + stress group (received saline, stress, and PTZ); and (6) MK-801 + stress group (received MK-801, stress, and PTZ). ... Pretreatment with MK-801 (0.125, 0.25, 0.50 mg/kg) significantly potentiated the protective effect of stress in PTZ-induced (65 mg/kg) seizures in both sexes by prolonging the onset of myoclonic jerks and clonic convulsions. Male mice had a significantly greater delay in the onset of myoclonic jerks (males, 66.7-295.5 sec; females, 54.0-247.5 sec; P < 0.05) and clonic convulsions (males, 123.5-789.8 sec; females, 94.5-757.2 sec; P < 0.05) compared with female mice in all groups (ie, PTZ control, stress, saline, MK-801, saline + stress, and MK-801 + stress groups). ... The findings of this study in mice suggest the involvement of sex hormones in the interaction between MK-801 pretreatment and acute CRS in PTZ-induced seizures. ... Adolescent male Wistar rats were exposed to EtOH vapor for 12 hr/d for 5 weeks. The effects of MK-801(0.0 to 0.1 mg/kg, intraperitoneally) on the electroencephalogram (EEG) and auditory event-related potentials (ERPs) were assessed after 8 weeks of abstinence from EtOH. ... Adolescent EtOH exposure reduced EEG variability in the frontal cortex in the 4 to 6 Hz band but had no effect on cortical and hippocampal EEG power and ERPs. ... MK-801 significantly reduced EEG power in the parietal cortex (4 to 6 Hz, 6 to 8 Hz, 8 to 16 Hz, 16 to 32 Hz) and hippocampus (16 to 32 Hz) and EEG variability in the parietal cortex (6 to 8 Hz, 16 to 32 Hz) following adolescent EtOH exposure. MK-801 produced a significant decrease in hippocampal EEG variability (4 to 6 Hz, 8 to 16 Hz, 16 to 32 Hz) in control, but not in EtOH-exposed rats. MK-801 reduced frontal P1 ERP amplitude and latency in response to the rare tone in EtOH-exposed rats compared to controls. In contrast, MK-801 significantly reduced P3 ERP amplitude and latency in control, but not in EtOH-exposed rats. /It was concluded that/ the effects of MK-801 on hippocampal EEG variability and P3 ERP amplitude and latency are significantly attenuated after a prolonged withdrawal period following adolescent EtOH exposure. However, the inhibitory effects of MK-801 on cortical and hippocampal EEG power were enhanced in rats exposed to EtOH during adolescence. Taken together, these data suggest protracted changes in NMDA systems following adolescent EtOH exposure. |
| 参考文献 |
|
| 其他信息 |
A potent noncompetitive antagonist of the NMDA receptor (RECEPTORS, N-METHYL-D-ASPARTATE) used mainly as a research tool. The drug has been considered for the wide variety of neurodegenerative conditions or disorders in which NMDA receptors may play an important role. Its use has been primarily limited to animal and tissue experiments because of its psychotropic effects.
Dizocilpine has a role as an anaesthetic, an anticonvulsant, a neuroprotective agent, a nicotinic antagonist and a NMDA receptor antagonist. It is a maleate salt and a tetracyclic antidepressant. It contains a dizocilpine(1+). A potent noncompetitive antagonist of the NMDA receptor (RECEPTORS, N-METHYL-D-ASPARTATE) used mainly as a research tool. The drug has been considered for the wide variety of neurodegenerative conditions or disorders in which NMDA receptors may play an important role. Its use has been primarily limited to animal and tissue experiments because of its psychotropic effects. The compound MK-801 [(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d] cyclohepten-5,10-imine maleate)] is a potent anticonvulsant that is active after oral administration and whose mechanism of action is unknown. We have detected high-affinity (Kd = 37.2 +/- 2.7 nM) binding sites for [3H]MK-801 in rat brain membranes. These sites are heat-labile, stereoselective, and regionally specific, with the hippocampus showing the highest density of sites, followed by cerebral cortex, corpus striatum, and medulla-pons. There was no detectable binding in the cerebellum. MK-801 binding sites exhibited a novel pharmacological profile, since none of the major neurotransmitter candidates were active at these sites. The only compounds that were able to compete for [3H]MK-801 binding sites were substances known to block the responses of excitatory amino acids mediated by the N-methyl-D-aspartate (N-Me-D-Asp) receptor subtype. These comprised the dissociative anesthetics phencyclidine and ketamine and the sigma-type opioid N-allylnormetazocine (SKF 10,047). Neurophysiological studies in vitro, using a rat cortical-slice preparation, demonstrated a potent, selective, and noncompetitive antagonistic action of MK-801 on depolarizing responses to N-Me-D-Asp but not to kainate or quisqualate. The potencies of phencyclidine, ketamine, SKF 10,047, and the enantiomers of MK-801 as N-Me-D-Asp antagonists correlated closely (r = 0.99) with their potencies as inhibitors of [3H]MK-801 binding. This suggests that the MK-801 binding sites are associated with N-Me-D-Asp receptors and provides an explanation for the mechanism of action of MK-801 as an anticonvulsant.[1] Whole-cell and single-channel recording techniques were used to study the action of the anticonvulsant drug MK-801 [(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]- cyclohepten-5,10-imine maleate) on responses to excitatory amino acids in rat neocortical neurons in cell culture. MK-801 caused a progressive, long-lasting blockade of current induced by N-methyl-D-aspartate (N-Me-D-Asp). However, during the time that N-Me-D-Asp responses were inhibited, there was no effect on responses to quisqualate or kainate, suggesting that N-Me-D-Asp receptors and kainate/quisqualate receptors open separate populations of ion channels. Binding and unbinding of MK-801 seems to be possible only if the N-Me-D-Asp-operated channel is in the transmitter-activated state: MK-801 was effective only when applied simultaneously with N-Me-D-Asp, and recovery from MK-801 blockade was speeded by continuous exposure to N-Me-D-Asp [time constant (tau) approximately equal to 90 min at -70 to -80 mV]. Recovery from block during continuous application of N-Me-D-Asp was strongly voltage dependent, being faster at positive potentials (tau approximately equal to 2 min at +30 mV). Mg2+, which is thought to block the N-Me-D-Asp-activated ion channel, inhibited blockade by MK-801 at negative membrane potentials. In single-channel recordings from outside-out patches. MK-801 greatly reduced the channel activity elicited by application of N-Me-D-Asp but did not significantly alter the predominant unitary conductance. Consistent with an open-channel blocking mechanism, the mean channel open time was reduced by MK-801 in a dose-dependent manner.[3] In summary, our work shows for the first time that the same reactivation parameters and pharmacological agent (MK-801) that disrupted the reconsolidation of a cocaine-associated memory for a CPP task did not disrupt reconsolidation of the memory for a self-administration task. Further, reactivation parameters that mimicked the self-administration procedure itself, and therefore should have promoted robust retrieval of the cocaine-associated memory, also failed to render this memory labile for disruption by MK-801. The possibility of diminishing persistent and unwanted memories by disrupting the reconsolidation process opens exciting new frontiers for developing treatments for pathological disorders, including drug abuse. However, the complexity of memory storage and subsequent memory retrieval that ultimately may lead to memory recoding has only begun to be elucidated and therefore requires further systematic investigation with regard to the timing and the specific parameters used for reactivation.[5] |
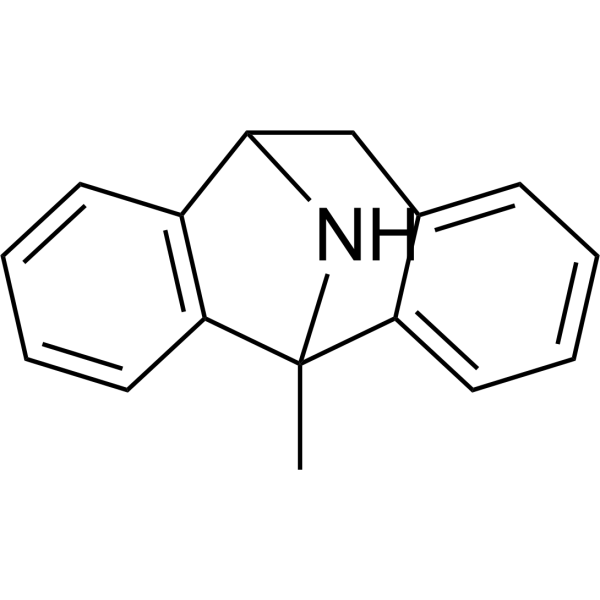
| 分子式 |
C16H15N
|
|---|---|
| 分子量 |
221.297
|
| 精确质量 |
221.12
|
| 元素分析 |
C, 86.84; H, 6.83; N, 6.33
|
| CAS号 |
70449-94-4
|
| 相关CAS号 |
77086-21-6; Dizocilpine maleate;77086-22-7;(-)-Dizocilpine maleate;121917-57-5
|
| PubChem CID |
1207
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.144g/cm3
|
| 沸点 |
320.3ºC at 760 mmHg
|
| 闪点 |
152.6ºC
|
| 折射率 |
1.632
|
| LogP |
3.479
|
| tPSA |
12.03
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
17
|
| 分子复杂度/Complexity |
313
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC12C3=CC=CC=C3CC(N1)C4=CC=CC=C24
|
| InChi Key |
LBOJYSIDWZQNJS-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C16H15N/c1-16-13-8-4-2-6-11(13)10-15(17-16)12-7-3-5-9-14(12)16/h2-9,15,17H,10H2,1H3
|
| 化学名 |
1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2,4,6,10,12,14-hexaene
|
| 别名 |
Neurogard; 70449-94-4; (+)-MK 801; 10,11-dihydro-5-methyl-5h-dibenzo[a,d]cyclohepten-5,10-imine; [3H]MK-801; MK-801; 5-methyl-10,11-dihydro-5H-5,10-epiminodibenzo[a,d][7]annulene; (Rac)-Dizocilpine;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.5188 mL | 22.5938 mL | 45.1875 mL | |
| 5 mM | 0.9038 mL | 4.5188 mL | 9.0375 mL | |
| 10 mM | 0.4519 mL | 2.2594 mL | 4.5188 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。