| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
Akt
|
|---|---|
| 体外研究 (In Vitro) |
AZD5363 是一种有效的 Akt 抑制剂,对 Akt1、Akt2 和 Akt3 的 IC50 分别为 3 nM、8 nM 和 8 nM。 [1] AZD5363 的效力约为 0.3 至 0.8 μM,可防止细胞中 AKT 底物的磷酸化。 AZD5363 的效力小于 < 3 μM,可阻止 182 种实体瘤和血液肿瘤细胞系中 41 种的生长。[2]显着预测 AZD5363 反应性的因素包括 PIK3CA 的激活突变、肿瘤抑制因子 PTEN 的丢失或失活以及 HER2 扩增。此外,细胞系的 RAS 突变状态与其对 AZD5363 的耐药性之间存在联系。[1]
为了了解化合物的选择性特征,对75种激酶的更大酶组进行了64/Capivasertib测定,其中35种也是AGC家族激酶。本文定义的显著活性是在1μM的固定浓度下抑制率>75%,仅观察到15种激酶,其中14种来自AGC家族,这并不奇怪。除Akt1–3外,还有ROCK2、MKK1、MSK1、MSK2、PKCγ、PKGα、PKGβ、PRKX、RSK2、RSK3、P70S6K和PKA。只有后两种激酶P70S6K和PKA受到抑制,其酶IC50值分别为6和7 nM,与Akt1–3的抑制值相当。然而,在这两种激酶的细胞终点,与主要的Akt药理学相比,活性相对降低。通过抑制TSC1缺失RT4膀胱癌症细胞中的S6磷酸化测定,细胞对P70S6K的IC50约为5μM,而通过抑制A431细胞中的VASP磷酸化测定对PKA的活性约为1μM。与ROCK2相比,针对相关ROCK1亚型的活性大大降低,IC50为470 nM。化合物64/Capivasertib在抑制多种细胞系中下游Akt底物的磷酸化方面也非常有效(表7)。对作为Akt细胞活性直接标志物的pGSK3β和pPRAS40有强烈的抑制作用。64的生长抑制作用也以标准增殖测定格式在182个肿瘤细胞系的更大的内部细胞组中进行了检测。敏感细胞系被定义为IC50为3μM或更低的抑制细胞系。大多数乳腺细胞系被证明是敏感的(64%),胃、子宫内膜、前列腺和血液系显示出中等敏感性(24-33%有反应)。对64的反应较差的细胞系来自肺(12%敏感)、结直肠(7%)和膀胱(0%)细胞。一条线的敏感程度可能与多种致癌标志物有关。具体而言,激活PIK3CA中的突变、肿瘤抑制因子PTEN的缺失或失活或HER2扩增都可以显著预测对治疗的反应性。此外,细胞系的RAS突变状态与64的抗性之间也存在相关性。[1] Capivasertib(AZD5363)是一种强效的AKT体外抑制剂[2] 在分离的酶试验中,AZD5363抑制了AKT的所有3种亚型,IC50<10 nmol/L。P70S6K和PKA的抑制效力与AKT亚型相似,但对Rho激酶ROCK1和ROCK2的抑制效力较低(表1)。通过在75种激酶中筛选浓度为1μmol/L的化合物,进一步了解了选择性,其中包括35种AGC激酶家族成员。AZD5363对15种激酶具有显著活性(在1μmol/L时抑制率>75%),其中14种是AGC家族的成员。这些酶是AKT1、AKT2、AKT3、P70S6K、PKA、ROCK2、MKK1、MSK1、MSK2、PKCγ、PKGα、PKGβ、PRKX、RSK2和RSK3(数据未显示)。 细胞中Capivasertib(AZD5363)的活性通过其抑制其底物PRAS40和GSK3β在BT474c(Her2+PIK3CA突变乳腺)和LNCaP(PTEN-null前列腺)癌症细胞中磷酸化的能力来确定,使用蛋白质印迹,并通过基于免疫荧光(Acumen)的测定来确定MDA-MB-468(PTEN-null-乳腺)癌症细胞中的活性。AZD5363在3个细胞系中抑制了这些底物的磷酸化,IC50值为0.06至0.76μmol/L(表1)。通过蛋白质印迹法在BT474c和LNCaP细胞中监测AKT的磷酸化状态以及信号网络中AKT下游的几种蛋白质。AZD5363有效地抑制了这些细胞系中S6和4E-BP1的磷酸化,而它增加了ser473和thr308处AKT的磷酸化(图2B)。AZD5363的活性也通过其诱导BT474c细胞中FOXO3a核转位的能力来测量。抑制AKT可防止FOXO3a的磷酸化;这导致FOXO3a易位到细胞核,在那里它能够开启p27、FasL和BIM等基因的表达,这些基因共同诱导细胞周期阻滞和/或凋亡。在BT474c细胞中,AZD5363诱导FOXO3a核转位,半最大有效浓度(EC50)值为0.69μmol/L;3μmol/L的浓度足以将FOXO3a几乎完全定位到细胞核中(图2C)。为了显示AZD5363在细胞中的P70S6K药理学,我们使用RT4膀胱癌症细胞系。这些细胞在TSC1中具有纯合缺失,TSC2表达非常低;因此,在这些细胞中,AKT在很大程度上与P70S6K脱钩。在该细胞系中,AZD5363抑制S6磷酸化,IC50值约为4.8μmol/L,而变构抑制剂MK-2206的活性要低得多(IC50>30μmol/L;补充图S1)。 Capivasertib(AZD5363)抑制一部分肿瘤细胞系的体外生长[2] 通过标准增殖试验,通过其抑制源自实体瘤和血液肿瘤的182个细胞系生长的能力来测量单一疗法AZD5363的活性。GI50<3μmol/L抑制的肿瘤细胞系被归类为敏感,而GI50>3μmol/L的肿瘤细胞株被归类为耐药。41个细胞系(23%)被归类为敏感;其中25株(14%)被GI50<1μmol/L抑制,被归类为高度敏感。乳腺癌细胞系的敏感性最高(14/22;64%);HER2+和ER+乳腺癌症细胞系始终敏感(图3A)。来自子宫内膜癌、胃癌、血液病和前列腺癌的细胞系均显示出24%至33%的反应频率,尽管仅筛选出6种独特的癌症细胞系。来源于肺和结直肠肿瘤的细胞系显示出较低的反应频率,分别为12%和7%,而所有来源于膀胱癌的细胞系都被归类为耐药。对AZD5363的敏感性与激活PIK3CA突变、PTEN缺失或失活突变或HER2扩增的存在之间似乎存在相关性。在25个被归类为高度敏感的细胞系中,有19个(76%)携带至少一种这些遗传缺陷,在41个被归类的敏感细胞系中有30个(73%)携带至少其中一种遗传缺陷(图3B)。当分析整个细胞组的数据时,无论是否存在其他突变,都发现PIK3CA突变的存在与AZD5363的敏感性之间存在显著关系(P=0.0059;t检验)。当分别分析PIK3CA螺旋和催化结构域的突变时,发现这两种突变类型与AZD5363的敏感性之间存在显著相关性(螺旋和激酶结构域突变分别为P=0.024和0.0047)。PTEN突变(缺失或基因序列突变)与AZD5363敏感性之间也存在显著相关性(P=0.0099;t检验;补充图S2)。还发现RAS突变的存在(共同分析K、N或H-RAS突变)与AZD5363的耐药性之间存在显著相关性(P=0.038;t检验)。当从分析中排除具有一致RAS突变的细胞系时,PIK3CA突变与AZD5363敏感性以及PTEN突变与AZD663敏感性之间的关系非常显著(两种情况下P<10−5;补充图S3)。 Capivasertib (AZD5363)的作用机制[2] 为了确定AZD5363是否具有主要的抗增殖或促凋亡作用机制,对一组乳腺癌和前列腺癌癌症细胞系进行了Sytox-Green测定,其在标准增殖测定中显示出对AZD5363的敏感性。Sytox Green测定法能够根据细胞数量和1μmol/L固定浓度下死细胞百分比的测定生成剂量-反应曲线。Sytox格林测定法中的GI50值通常与增殖测定值相似;2次检测的值均在5倍以内,14个细胞系中有8个(57%)在2倍以内。然而,在与1μmol/L AZD5363孵育后,14个细胞系中只有3个细胞死亡超过10%;这些细胞系是BT474c乳腺癌和2个前列腺癌症细胞系(LNCaP和PC346C-Flut1;补充图S4A)。在BT474c细胞系中,通过将细胞暴露于浓度逐渐升高的AZD5363并监测切割的半胱氨酸天冬氨酸蛋白酶-3和切割的PARP,证实了细胞死亡的诱导。在48小时时,在BT474c细胞中观察到这两种凋亡标志物的剂量成比例增加。 |
| 体内研究 (In Vivo) |
裸鼠口服Capivasertib (AZD5363)(100, 300 mg/kg) 会导致 U87 中血糖水平可逆性升高,2[18F]氟-2-脱氧-d-葡萄糖 (18F-FDG) 摄取呈剂量依赖性下降-MG 异种移植物,以及 BT474c 异种移植物中 PRAS40、GSK3 和 S6 磷酸化的剂量依赖性减少。长期口服 AZD5363(130、200 和 300 mg/kg)会对各种肿瘤类型(包括曲妥珠单抗耐药 HER2+ 乳腺癌模型)的异种移植物产生剂量依赖性生长抑制。此外,在乳腺癌异种移植物中,AZD5363 显着增加多西他赛、拉帕替尼和曲妥珠单抗的抗肿瘤活性。 [2]
首先通过测量BT474c乳腺腺癌异种移植物模型中的药效活性来表征64/Capivasertib在体内的作用。在单次口服100和300mg/kg剂量后,64以与血浆暴露直接相关的方式有效抑制了Akt下游底物pGSK3β和pPRAS40以及pS6的磷酸化(图4)。pPRAS40和pGSK3β的抑制作用持续4小时,8小时后开始恢复,24小时后化合物被消除,恢复到基础水平。更远端的细胞标记物pS6显示出类似的暴露反应,尽管总体上抑制作用较小。在14天的同一模型中,还评估了连续口服64对肿瘤生长的影响。当以200mg/kg的剂量每天给药一次时,64的效果不如以100mg/kg的剂量每天两次(39%的抑制率对80%)。每天两次200mg/kg的剂量对生长的抑制作用最大,导致104%的抑制,这被证明是最大耐受性良好的连续每日两次剂量(图5)。[1] Capivasertib(AZD5363)抑制体内人类肿瘤异种移植物的生长[2] 通过连续口服给药裸鼠来确定单药AZD5363对异种移植物生长的影响。在所有测试的模型中都观察到剂量依赖性抑制。在HER2+扩增的PIK3CA突变体BT474c异种移植物中,每天两次口服100mg/kg可产生80%的抑制作用(P<0.0001);该方案比每天200mg/kg更有效(39%抑制;P=0.02),但不如每天两次200mg/kg的最大耐受剂量有效(104%抑制;P<0.0001;图4A)。在HER2+扩增的PIK3CA突变HCC-1954乳腺癌症异种移植物中,每天两次150 mg/kg的AZD5363导致显著的肿瘤消退(129%抑制;P<0.0001),而每天两次75 mg/kg导致111%抑制(P<0.001;图4B)。相比之下,30 mg/kg的曲妥珠单抗每周两次在HER2+模型中没有活性。在786-0 PTEN-null癌症肾脏异种移植物中,每天两次150 mg/kg的AZD5363导致部分消退(125%抑制;P<0.0001),而每天两次75 mg/kg导致部分生长抑制(56%;P=0.001;图4C)。AZD5363还抑制PIK3CA突变株/PTEN-null HGC-27胃癌症异种移植物的生长,每天两次,剂量超过50mg/kg;在该模型中,当剂量超过100mg/kg时,每天两次观察到轻微的肿瘤消退,在停止给药后观察到剂量依赖性的进展时间(108%、106%和72%的生长抑制;150、100和50mg/kg每天两次的剂量分别为P<0.0001、0.001和0.003;图4D)。 Capivasertib (AZD5363)在体内具有药效活性[2] 在裸小鼠BT474c异种移植物中,在300和100mg/kg的急性剂量后,测定了AZD5363的药效活性,并与血浆药代动力学相关(图5A)。在300mg/kg剂量的AZD5363后,PRAS40、GSK3β和S6的磷酸化被显著抑制至少24小时。pPRAS40的抑制最为强烈,在1小时和2小时时抑制率约为90%,在24小时时恢复到约70%的抑制率。GSK3β和S6磷酸化的抑制率从1小时的约80%变化到8小时的约50%和24小时的约40%。AZD5363的总血浆暴露量(未校正蛋白质结合)在1小时时超过10μmol/L,在300mg/kg剂量后约8小时内保持在1μmol/L以上。在100 mg/kg剂量的AZD5363后,所有3种生物标志物的磷酸化在至少8小时内受到显著抑制,但抑制程度小于300 mg/kg剂量后观察到的抑制程度(图5A)。在100 mg/kg剂量后,AZD5363的血浆暴露量约为1μmol/L,持续至少4小时。绘制单个动物PRAS40磷酸化之间的药效学-药代动力学关系图表明,在总血浆暴露量约为0.1μmol/L AZD5363时,pPRAS40的抑制率为50%(图5B)。在本研究中使用的非致死动物中,AZD5363剂量与血糖浓度之间也存在剂量和时间依赖关系;在300mg/kg剂量后2小时,葡萄糖浓度增加到约20mmol/L,16小时后回落到对照水平,而在100mg/kg剂量后,葡萄糖浓度上升不到2倍,8小时后降至对照水平(图5C)。 AKT在葡萄糖代谢中起着关键作用;其底物GSK3β和AS160可以分别调节糖原合成和葡萄糖转运蛋白功能,通过该途径的信号传导可以调节糖酵解酶,包括己糖激酶和磷酸果糖激酶。事实上,AKT激活可能至少部分解释了沃伯格效应——肿瘤中从氧化磷酸化到糖酵解升高的代谢转变。因此,18F-FDG-PET成像有可能成为AKT抑制后通路输出的生物标志物。急性给药4小时后,在禁食裸小鼠的U87-MG异种移植物中测试了Capivasertib (AZD5363)对18F-FDG摄取的影响。与赋形剂对照组相比,130、200和300 mg/kg AZD5363的剂量均导致18F-FDG的肿瘤摄取显著降低,但血糖浓度仅在200和300 g/kg剂量后的这一时间点显著升高(图6A)。 鉴于似乎有可能在体外诱导BT474c细胞系的细胞死亡,我们比较了连续和间歇给药方案的Capivasertib (AZD5363)对BT474c异种移植物体内生长的影响,该方案提供了相似的曲线下面积(AUC),但Cmax值不同。如前所述,每天两次连续给药100 mg/kg,达到约1μmol/L AZD5363的稳态暴露,导致肿瘤生长抑制率超过90%,但没有肿瘤消退(补充图S4B)。然而,每4天服用300 mg/kg,3天休息,达到超过10μmol/L AZD5363的4峰浓度,导致给药期间肿瘤消退,随后在药物假期期间肿瘤生长恢复。当在短期慢性给药(3天给药)后对这些异种移植物进行药效学分析时,在300mg/kg剂量的AZD5363给药后2小时,切割的半胱氨酸天冬氨酸蛋白酶-3显著诱导,而在每天两次100mg/kg剂量给药后没有观察到这一点。相比之下,在两种药物给药后8小时,Ki-67染色显著降低(补充图S4B)。这些实验表明,在由于AKT抑制而易发生凋亡的肿瘤中,高剂量、间歇性的AZD5363方案可能比连续方案更有效。 Capivasertib(AZD5363)在体内增强HER2抑制剂和多西他赛的活性[2] 在HER2+、PIK3CA突变体KPL4乳腺癌症异种移植物中测试了AZD5363与治疗性抗体和HER2信号小分子抑制剂结合的潜力。该模型显示了拉帕替尼和曲妥珠单抗的次优反应。每天100 mg/kg的单药拉帕替尼、每周两次的15 mg/kg曲妥珠单抗和每天两次的150 mg/kg AZD5363均抑制了肿瘤生长(分别为37%,不显著;69%,P=0.002;65%,P=0.004),但均未达到停滞状态。相比之下,AZD5363和曲妥珠单抗或AZD5363与拉帕替尼的组合具有良好的耐受性,分别导致107%和109%的肿瘤消退(P<0.0001)。此外,与单药治疗组相比,两种组合在停止给药后都显示出增强的生长延迟(图7A)。AZD5363与多烯紫杉醇的组合在2种不同的癌症异种移植物BT474c和HCC-1187中进行了测试。在BT474c异种移植物中,单剂量15mg/kg多西他赛导致肿瘤轻微消退(129%抑制;P<0.0001)。当与150 mg/kg每日两次的AZD5363联合使用时,肿瘤显示出显著且大大增强的消退(159%的抑制率;P<0.0001),9个肿瘤中有6个显示出完全消退,在实验结束时肿瘤无法测量(图7B)。在HCC-1187异种移植物模型中评估了将5mg/kg多西他赛与AZD5363的每周给药周期联合使用的效果。在第一个实验中(图7C),研究了150 mg/kg每日两次的Capivasertib (AZD5363)和多西他赛的组合。该组合比各自的单一治疗组有效得多(100%抑制;联合治疗的P=0.0003,而单一治疗的多西他赛为71%P=0.002,单一治疗的AZD5363为79%P=0.005),并显示出切割的半胱氨酸天冬氨酸蛋白酶-3染色增加了凋亡的证据(补充图S6)。在第二个实验中(图7D),研究了5 mg/kg每周一次多西他赛与2种AZD5363方案的组合,这些方案可提供等效的AUC。这两种方案都提高了多西他赛单一疗法的疗效,且程度相似;在第一个给药期结束时,多西他赛单一疗法对肿瘤生长的抑制率为76%(P=0.0003),而多西他赛与AZD5363的连续和间歇给药方案的组合分别对肿瘤生长抑制了103%和101%。停止给药时,所有治疗组均出现再生。在用相同的治疗方法再次挑战后,多西他赛单药治疗的肿瘤大小缓慢增加,而联合治疗组则显示出渐进性消退。多西他赛与300 mg/kg AZD5363的间歇给药方案(4天服用,3天休息)的组合最初似乎略优于多西他赛和100 mg/kg AZD5363/天两次连续给药方案的组合,但在实验结束时,各组规模没有显著差异(图7D)。 |
| 酶活实验 |
通过 Caliper 片外孵化迁移率变化测定评估 Capivasertib (AZD5363)和其他化合物抑制 AKT1、AKT2 和 AKT3 活性的能力。将活性重组 AKT1、AKT2 或 AKT3 与 5-FAM 标记的定制合成肽底物以及浓度不断增加的抑制剂一起孵育。最终反应包含 1 至 3 nM AKT1、AKT2 或 AKT3 酶、1.5 mM 肽底物、每个 AKT 同工型的 Km ATP、10 mM MgCl2、4 mM DTT、100 mM HEPES 和 0.015% Brij-35。反应在室温下进行一小时,然后添加含有 40 mM EDTA、5% DMSO、0.1% 涂层试剂、0.1% Brij-35 溶液和 100 mM HEPES 的缓冲液来停止反应。之后,在 Caliper LC3000 上检查板,从而实现肽底物和磷酸化产物的电泳分离,以及随后激光诱导荧光的检测和定量。
酶测定[2] 通过Caliper芯片外培养迁移率转移试验评估了Capivasertib(AZD5363)抑制AKT1、AKT2和AKT3活性的能力。将活性重组AKT1、AKT2或AKT3与5-FAM标记的定制合成肽底物(剑桥研究生化)以及浓度逐渐增加的抑制剂一起孵育。最终反应含有1至3nmol/L的AKT1、AKT2或AKT3酶;1.5μmol/L肽底物;每种AKT亚型Km处的ATP;10 mmol/L氯化镁、4 mmol/L二硫苏糖醇(DTT)、100 mmol/L HEPES和0.015%Brij-35。在室温下孵育反应1小时,并通过加入含有100 mmol/L HEPES、0.015%Brij-35溶液、0.1%包被试剂、40 mmol/L EDTA和5%DMSO的缓冲液来停止反应。然后使用Caliper LC3000分析板,通过电泳分离肽底物和磷酸化产物,随后检测和定量激光诱导荧光。为了确定激酶的选择性,还对Capivasertib (AZD5363)对PKA、ROCK1、ROCK2和P70S6K进行了测试。如前所述,PKA、ROCK1和ROCK2活性通过Caliper芯片外培养迁移率变化测定法测定。用于测量ROCKI活性的最终反应条件为5 nmol/L活性重组ROCK1、1.5μmol/L异硫氰酸荧光素(FITC)标记的定制肽底物、7μmol/L ATP、1 mmol/L DTT、5 mmol/L MgCl2、100 mmol/L HEPES、0.015%Brij-35和5 mmol/Lβ-甘油磷酸;用于测量ROCK2活性的最终反应包含7.5 nmol/L活性重组ROCK2、1.5μmol/L FAM标记的定制肽底物、7.5μmol/L ATP、1 mmol/L DTT、10 mmol/L MgCl2、100 mmol/L HEPES、0.015%Brij-35和5 mmol/Lβ-甘油磷酸;在含有0.0625 nmol/L PKA、3μmol/L FITC标记的定制肽底物、4.6μmol/L ATP、1 mmol/L DTT、10 mmol/L MgCl2、110 mmol/L HEPES和0.015%Brij-35的最终反应中测量蛋白激酶A(PKA)活性。P70S6K活性通过放射性(33P-ATP)滤膜结合试验测量。重组S6K1(T412E)针对底物肽(KKRNRTLTV)进行了检测,最终体积为25.5μL,含有8 mmol/L MOPS、200μmol/L EDTA、100μmol/L底物肽、10 mmol/L乙酸镁、20μmol/Lγ-33P-ATP(50-1000cpm/pmol)和浓度逐渐增加的Capivasertib (AZD5363)。在室温下孵育反应30分钟,并通过加入0.5mol(3%)正磷酸终止反应。然后将反应物收集到P81 UniFilter上,并对产物形成进行定量。通过拟合Origin 7.0中的数据获得所有酶测定的IC50值。为了评估更广泛的选择性,在Dundee激酶小组中也对Capivasertib(AZD5363)进行了测试。 |
| 细胞实验 |
MTS 和 Sytox Green 是用于测量细胞增殖的两种方法。简而言之,将细胞铺板于 96 孔培养皿中,并在 37°C、5% CO2 下孵育整夜。随后,将细胞置于浓度范围为 30 至 0.003 μM 的 ACapivasertib (AZD5363) 中 72 小时。根据制造商的说明,使用 CellTiter 水性非放射性细胞增殖测定试剂评估细胞增殖的 MTS 终点。 Sytox Green 终点,Sytox Green 核酸染料以 0.13 μM 的终浓度对细胞进行染色,并使用 Acumen Explorer 计算死细胞的数量。皂苷透化(最终浓度为 0.03%,在 TBS-EDTA 缓冲液中稀释)后,将细胞孵育过夜以确定总细胞计数。对 Sytox Green 和 MTS 终点进行给药前测量,并使用活细胞计数或 MTS 吸光度读数计算将处理的细胞生长与未处理的细胞生长相比减少一半所需的浓度。
AKT的细胞抑制[2] 使用MDA-MB-468乳腺癌症细胞系开发了基于高通量筛选细胞的测定法来测量细胞AKT活性。细胞暴露于浓度范围为3至0.003μmol/L的Capivasertib(AZD5363)。处理2小时后,用甲醛固定细胞,洗涤,用0.5%聚山梨酯20渗透,然后用针对GSK3βser9的磷酸特异性抗体进行探测。用Acumen Explorer激光扫描细胞仪(TTP LabTech)测量磷酸化GSK3βser9的水平,并通过拟合Origin 7.0中的数据估算IC50值。 蛋白质印迹分析[2] LNCaP前列腺癌症细胞和BT474c乳腺癌细胞暴露于浓度范围为10至0.03μmol/L的Capivasertib(AZD5363)中2或24小时。然后用含有25 mmol/L Tris-HCl、3 mmol/L EDTA、3 mmol/L EGTA、50 mmol/L NaF、2 mmol/L原钒酸钠、0.27 mol/L蔗糖、10 mmol/Lβ-甘油磷酸、5 mmol/L焦磷酸钠和0.5%Triton X-100以及蛋白酶和磷酸酶抑制剂的缓冲液在冰上裂解细胞。然后用样品加载缓冲液稀释裂解物,在4%至12%的Bis-Tris-Novex凝胶上分离,转移到硝化纤维膜上,并用磷酸-PRAS40、磷酸-GSK3β、磷酸-S6、磷酸-AKT、磷酸-4E-BP1、总4E-BP1,PARP和半胱氨酸天冬氨酸蛋白酶-3的抗体进行检测。与第一抗体孵育过夜后,洗涤膜并用辣根过氧化物酶标记的第二抗体孵育,然后在Syngene ChemiGenius上用SuperSignal West Dura化学发光底物显示免疫印迹蛋白。使用Syngene GeneTools进行定量,并估算IC50值。 FOXO3a易位分析[2] 将BT474c细胞接种到透明底部、黑色壁96孔板中,在37°C、5%CO2下孵育过夜,然后暴露于浓度范围为3至0.003μmol/L的Capivasertib(AZD5363)。处理2小时后,用甲醛固定细胞,用0.5%聚山梨酯20渗透,然后在4°C下用抗FOXO3a的第一抗体探测过夜。洗涤步骤后,用与Alexa Fluor 488染料偶联的第二抗体孵育细胞,并使用Cellomics ArrayScan进行成像。开发了一种测量核质荧光强度比的算法,并估算了IC50值。 增殖试验[2] 细胞增殖试验采用MTS和Sytox Green两种方法测定。简而言之,将细胞接种在96孔板中(密度允许在72小时的测定中对数生长),并在37°C、5%CO2下孵育过夜。然后将细胞暴露于浓度范围为30至0.003μmol/L的Capivasertib(AZD5363)中72小时。对于MTS终点,根据制造商的方案,使用CellTiter AQueous非放射性细胞增殖测定试剂测量细胞增殖。使用Tecan Ultra仪器测量吸光度。对于Sytox Green终点,将稀释在TBS-EDTA缓冲液中的Sytox绿色核酸染料加入细胞中(终浓度为0.13μmol/L),并使用Acumen Explorer检测死细胞数量。然后通过加入皂苷(终浓度为0.03%,在TBS-EDTA缓冲液中稀释)使细胞透性,孵育过夜并测量总细胞计数。对MTS和Sytox-Green终点进行了给药前测量,并使用吸光度读数(MTS)或活细胞计数确定了将处理细胞的生长减少到未处理细胞的一半所需的浓度(GI50)值。 |
| 动物实验 |
Mice: Specific, pathogen-free, female nude mice (nu/nu: Alpk) and male SCID mice (SCID/CB17; 786-0 xenograft studies) are used. The mice are randomly assigned to control and treatment groups once the mean tumor sizes reach about 0.2 cm3. The treatment groups received RP-56976, which was dissolved in 2.6% ethanol in injectable water, once on day 1, at 15 or 5 mg/kg once a week, and Capivasertib (AZD5363), which was dissolved in a 10% DMSO 25% w/v Kleptose HPB (Roquette) buffer by oral gavage. When used in conjunction with Capivasertib (AZD5363), RP-56976 is given an hour before the oral dose. The control group received the DMSO/Kleptose buffer alone, twice daily by oral gavage. For the duration of the study, tumor volumes (as determined by caliper), animal weight, and tumor condition are noted twice a week. By using CO2 euthanasia, mice are sacrificed. Using the formula: (length×width)×√(length×width)×(π/6), the tumor volume is calculated by considering length to be the longest diameter across the tumor and width to be the corresponding perpendicular diameter. By comparing the variations in tumor volume between the control and treated groups, growth inhibition from the onset of treatment is evaluated.
Implantation of cells into mice [2] For in vivo implants, cells were harvested from T225 tissue culture flasks by a 2- to 5-minute treatment with 0.05% trypsin (Invitrogen) in EDTA solution followed by suspension in basic medium and 3 washes in PBS. Only single-cell suspensions of greater than 90% viability, as determined by trypan blue exclusion, were used for injection. Tumor cells were injected subcutaneously in the left flank of the animal in a volume of 0.1 mL. For BT474c studies the animals were supplemented with 0.36 mg/60-d 17β estradiol pellets 1 day before cell implantation. For KPL-4 and HGC-27 antitumor studies, tumors were passaged as approximately 10 mm3 fragments into the flank before carrying out efficacy studies, to achieve more consistent take rates. Efficacy studies [2] When mean tumor sizes reached approximately 0.2 cm3, the mice were randomized into control and treatment groups. The treatment groups received varying dose schedules of Capivasertib (AZD5363) solubilized in a 10% DMSO 25% w/v Kleptose HPB buffer by oral gavage, docetaxel solubilized in 2.6% ethanol in injectable water by intravenous injection once on day 1 at 15 or 5 mg/kg once weekly. When administered in combination, docetaxel was administered 1 hour before the oral dose of Capivasertib (AZD5363). The control group received the DMSO/Kleptose buffer alone, twice daily by oral gavage. Tumor volumes (measured by caliper), animal body weight, and tumor condition were recorded twice weekly for the duration of the study. Mice were sacrificed by CO2 euthanasia. The tumor volume was calculated (taking length to be the longest diameter across the tumor and width to be the corresponding perpendicular diameter) using the formula: (length × width) × √(length × width) × (π/6). Growth inhibition from the start of treatment was assessed by comparison of the differences in tumor volume between control and treated groups. Because the variance in mean tumor volume data increases proportionally with volume (and is therefore disproportionate between groups), data were log transformed to remove any size dependency before statistical evaluation. Statistical significance was evaluated using a one-tailed, 2-sample t test. Pharmacodynamic studies [2] When mean tumor size reached 0.5 cm3, the mice were randomized into control (n = 8 animals) and treatment groups (n = 5 animals per group). The treatment groups received 300 or 100 mg/kg acute dose of Capivasertib (AZD5363)solubilized in a DMSO/Kleptose buffer, by oral gavage . The control group received the DMSO/Kleptose buffer alone, once by oral gavage. At 2, 4, 8, 16, or 24 hours after dosing, the animals were humanely killed and the tumor was snap frozen in liquid nitrogen and stored at −80°C. Total blood was collected by intracardiac puncture and plasma prepared and immediately frozen at −20°C for pharmacokinetic analysis. Frozen tumors were homogenized using Fastprep methodology lysis matrix A and lysates generated using adjusted lysis buffer (1% Triton X-100). Equivalent amounts of protein (12 μg per lane) were resolved by 4% to 15% gradient SDS-PAGE premade gels and transferred to nitrocellulose membranes. Radiotracer preparation and positron emission tomography imaging [2] 2[18F]Fluoro-2-deoxy-d-glucose (18F-FDG) was supplied by PETNET solutions. Specific activity was approximately 185 GBq/mmol. Radiochemical purity (determined by thin-layer chromatography) was greater than 95%. Imaging was carried out using the Inveon positron emission tomography (PET) and computed tomography-docked system from Siemens Medical Solutions. Data were acquired with IAW software Version 1.4.3 and analyzed with IRW software version 3.0. The performance of the scanner has been documented previously. Images were reconstructed using the MAP/3D algorithm (quality control measurement was carried out for the PET scanner before commencement of imaging). Following imaging, tumors were snap frozen in liquid nitrogen and stored at −80°C. Mice received on average 7.96 MBq of radioactivity as a bolus intravenous injection via the tail vein under isoflurane anesthesia. Food was withdrawn on the day of imaging in half-hour intervals so each mouse was fasted for 4 hours before injection of 18F-FDG. Mice were dosed with either vehicle or Capivasertib (AZD5363) (130, 200, or 300 mg/kg) and were imaged 4 hours after drug dosing. Mice were anesthetized in preparation for 18F-FDG injection 3 hours and 15 minutes after dosing, and anesthesia was then maintained for a 45-minute uptake period followed by a 20-minute PET scan under isoflurane anesthesia. Anesthesia was induced using isoflurane delivered in 100% oxygen (∼1.5% isoflurane, 3 L oxygen). Respiration and temperature were monitored throughout, with body temperature being maintained at 36°C–37°C. Following imaging, tumors were snap frozen in liquid nitrogen and stored at −80°C. Images were reconstructed using the MAP/3D algorithm (quality control measurement was carried out for the PET scanner before commencement of imaging). Regions of interest (ROI) were manually drawn on the 3-dimensional visualization package of Inveon Research Workplace software, to determine radioactivity uptake in the whole tumor. Data were expressed as the maximum standardized uptake value (SUV). Max SUV was calculated using the following formula described by Gambhir, where ID is the injected dose. Blood glucose concentration was measured before vehicle or Capivasertib (AZD5363) dosing and after PET scanning. Blood glucose concentrations were measured with an Accu Chek meter. Data are reported as mean ± SEM unless otherwise stated. Statistical analyses were conducted using GraphPad prism (v. 4.02). An ANOVA allowing for treatment group was carried out as well as group means, which were compared using a 2-sided t test. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The capivasertib steady-state AUC is 8,069 h·ng/mL (37%) and Cmax is 1,371 ng/mL (30%). Steady-state concentrations are predicted to be attained on the 3rd and 4th dosing day of each week, starting week 2. Capivasertib plasma concentrations are approximately 0.5% to 15% of the steady-state Cmax during the off-dosing days. Capivasertib AUC and Cmax are proportional with doses over a range of 80 to 800 mg (0.2 to 2 times the approved recommended dosage). Tmax is approximately 1-2 hours. The absolute bioavailability is 29%. No clinically meaningful differences in capivasertib pharmacokinetics were observed following the administration of capivasertib with a high-fat meal (approximately 1,000 kcal; fat 60%) or a low-fat meal (approximately 400 kcal; fat 26%). Following a single radiolabeled oral dose of 400 mg, the mean total recovery was 45% from urine and 50% from feces. The steady-state oral volume of distribution is 1,847 L (36%). The steady-state oral clearance of capivasertib is 50 L/h (37% CV), and renal clearance was 21% of total clearance. Metabolism / Metabolites Capivasertib is primarily metabolized by CYP3A4 and UGT2B7. Biological Half-Life The half-life of capivasertib is 8.3 hours. Pharmacokinetic Profiling [1] The DMPK profile of 64/Capivasertib is highlighted in Table 6. Protein binding remained low across all species, consistent with initial lead 3. Compound 64/Capivasertib is extensively distributed outside of blood, with volumes of distribution ranging from 2 to 4 L/kg in preclinical species. Oral bioavailability in mouse remains high despite higher clearance, which may indicate a saturation of first-pass metabolism with the oral dose or extrahepatic metabolism. The profile in rat is somewhat worse, however: whole blood clearance is relatively high, and consequently bioavailability remains a modest 13%. Optimization of the critical parameters of cell potency, ROCK selectivity, and absolute hERG margin of 3 has been achieved, but here at the expense of some of the favorable pharmacokinetic properties the early lead demonstrated. The profile in dog appears more balanced, with moderate clearance and moderate bioavailability. As with the initial lead, in vitro intrinsic hepatic clearance (Clint) measured in hepatocytes is generally low, with turnover in human cells only measurable by an assay with a 2 h incubation. Plasma exposure of Capivasertib (AZD5363) was approximately 1 μmol/L for at least 4 hours following a 100 mg/kg dose. Plotting the pharmacodynamic–pharmacokinetic relationship between PRAS40 phosphorylation of individual animals showed that 50% inhibition of pPRAS40 occurred at a total plasma exposure of approximately 0.1 μmol/L AZD5363 (Fig. 5B). A dose- and time-dependent relationship between dose of AZD5363 and blood glucose concentration was also seen in the nonfasted animals used for this study; the glucose concentration increased to approximately 20 mmol/L at 2 hours after a 300 mg/kg dose, and fell back to control levels by 16 hours whereas the glucose concentration increased by less than 2-fold following a 100 mg/kg dose, and fell to control levels by 8 hours (Fig. 5C).[2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Capivasertib plasma protein binding is 22% and the plasma-to-blood ratio is 0.71. Hepatotoxicity In the prelicensure trials of capivasertib in combination with fulvestrant as therapy of advanced or metastatic breast cancer, liver test abnormalities were frequent, with elevations in ALT levels in 23% compared to 13% of those on placebo and fulvestrant. However, the enzyme elevations were usually transient, mild-to-moderate in severity and not associated with symptoms or jaundice. ALT elevations above 5 times the upper limit of normal (ULN) arose in 3% of patients on capivasertib vs 0% with placebo. Because capivasertib was always given in combination with a hormonal agent, it was not always possible to attribute the liver test abnormalities to capivasertib alone. There were no discontinuations of capivasertib because of liver test abnormalities and no episodes of clinically apparent liver injury or deaths from liver failure. Since approval and clinical availability of capivasertib, there have been no published case reports of clinically apparent liver injury with jaundice, but clinical experience with its use has been limited. Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of capivasertib during breastfeeding. Because of its potential toxicity in the breastfed infant, the manufacturer recommends that breastfeeding be discontinued during capivasertib therapy. With a half-life of 8.3 hours the drug should be cleared from the maternal bloodstream in 2 days. However, capivasertib is used in combination with fulvestrant, which may increase the risk to the infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| 参考文献 | |
| 其他信息 |
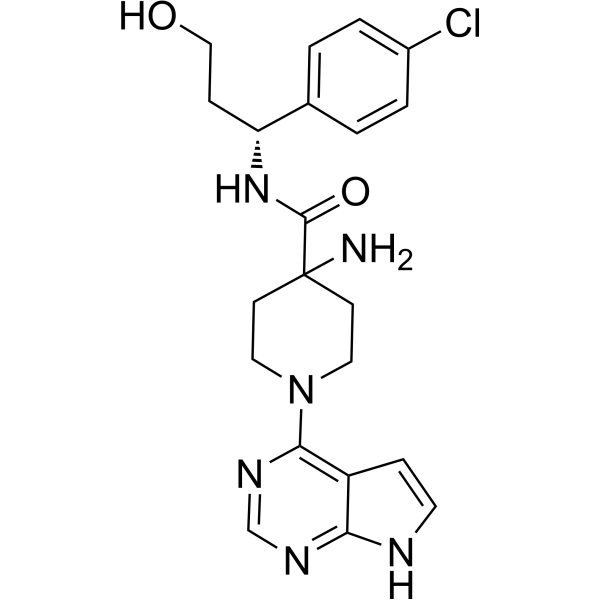
Capivasertib is an aminopiperidine that is piperidine substituted by 7H-pyrrolo[2,3-d]pyrimidin-4-yl, amino, and [(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]aminocarbonyl groups at positions 1, 4, and 4, respectively. It is a pan-AKT kinase inhibitor used in combination with fulvestrant for the treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, locally advanced or metastatic breast cancer with one or more PIK3CA/AKT1/PTEN-alterations. It has a role as an antineoplastic agent and an EC 2.7.11.1 (non-specific serine/threonine protein kinase) inhibitor. It is a pyrrolopyrimidine, an aminopiperidine, a piperidinecarboxamide, a member of monochlorobenzenes, a primary alcohol and a secondary carboxamide.
Hormone receptor (HR) positive, especially estrogen receptor-positive, HER2-negative breast cancer is the most common subtype of metastatic breast cancer, resulting in more than 400,000 deaths annually. Although endocrine-based therapy is the first line of treatment, resistance eventually emerges, leaving chemotherapy the only but often ineffective treatment left. Therefore, significant research has been put into developing genetically targeted treatments. The PIK3/AKT pathway is one of the most commonly activated pathways in breast cancer, mainly through the constitutively active mutation in AKT1, loss of function mutation in PTEN, a negative regulator of the PIK3/AKT pathway, or PIK3CA mutations. Therefore, targeting the PIK3/AKT pathway presents a promising approach for the treatment of breast cancer, leading to the development of capivasertib, a pan-AKT kinase inhibitor. On November 17th, 2023, capivasertib, under the brand name TRUQAP, was approved by the FDA for the treatment of adult patients HR-positive, HER2-negative locally advanced or metastatic breast cancer with one or more alterations in PIK3CA/AKT1/PTEN gene(s) in combination with [fulvestrant]. This approval is based on favorable results obtained from the CAPItello-291 trial, where the combination of capivasertib and [fulvestrant] reduced the risk of disease progression or death by 50% versus [fulvestrant] alone. Capivasertib is a novel pyrrolopyrimidine derivative, and an orally available inhibitor of the serine/threonine protein kinase AKT (protein kinase B) with potential antineoplastic activity. Capivasertib binds to and inhibits all AKT isoforms. Inhibition of AKT prevents the phosphorylation of AKT substrates that mediate cellular processes, such as cell division, apoptosis, and glucose and fatty acid metabolism. A wide range of solid and hematological malignancies show dysregulated PI3K/AKT/mTOR signaling due to mutations in multiple signaling components. By targeting AKT, the key node in the PIK3/AKT signaling network, this agent may be used as monotherapy or combination therapy for a variety of human cancers. Drug Indication Capivasertib, in combination with fulvestrant, is indicated for the treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, locally advanced or metastatic breast cancer with one or more PIK3CA/AKT1/PTEN-alteration as detected by an FDA-approved test following progression on at least one endocrine-based regimen in the metastatic setting or recurrence on or within 12 months of completing adjuvant therapy. Treatment of breast cancer , Treatment of prostate cancer Mechanism of Action Capivasertib is an inhibitor of all 3 isoforms of serine/threonine kinase AKT (AKT1, AKT2, and AKT3) and inhibits phosphorylation of downstream AKT substrates. AKT activation in tumors is a result of activation of upstream signaling pathways, mutations in AKT1, loss of phosphatase and tensin homolog (PTEN) function, and mutations in the catalytic subunit alpha of phosphatidylinositol 3-kinase (PIK3CA). Pharmacodynamics In vitro, capivasertib reduced the growth of breast cancer cell lines including those with relevant PIK3CA or AKT1 mutations or PTEN alteration. In vivo, capivasertib alone and in combination with fulvestrant inhibited tumor growth of mouse xenograft models including estrogen receptor-positive breast cancer models with alterations in PIK3CA, AKT1, and PTEN. The exposure-response relationship and time course of pharmacodynamic response for the effectiveness of capivasertib has not been fully characterized. Exposure-response relationships were observed for diarrhea (CTCAE Grade 2 to 4), rash (CTCAE Grade 2 to 4), and hyperglycemia (CTCAE Grades 3 or 4) at doses of 80 to 800 mg (0.2 to 2 times the approved recommended dosage). At the recommended capivasertib dose, a mean increase in the QTc interval > 20 ms was not observed. Wide-ranging exploration of analogues of an ATP-competitive pyrrolopyrimidine inhibitor of Akt led to the discovery of clinical candidate AZD5363, which showed increased potency, reduced hERG affinity, and higher selectivity against the closely related AGC kinase ROCK. This compound demonstrated good preclinical drug metabolism and pharmacokinetics (DMPK) properties and, after oral dosing, showed pharmacodynamic knockdown of phosphorylation of Akt and downstream biomarkers in vivo, and inhibition of tumor growth in a breast cancer xenograft model. Compound 3 served as a lead Akt inhibitor with an acceptable DMPK profile in preclinical species and in vivo antitumor efficacy with modulation of biomarkers following oral dosing. Nevertheless, it had an unfavorably low ROCK selectivity, only modest cell activity, and unwanted activity at the hERG ion channel. A crystal structure of this compound bound to Akt1 suggested a possible vector for further substitution, and this position was ultimately explored with a range of diverse substituents and chain lengths, leading ultimately to compound 64, AZD5363. This agent inhibits all Akt isoforms with a potency of <10 nM in vitro and is a potent inhibitor of phosphorylation of the Akt substrates GSK3β, PRAS40, and S6 in a range of cell lines. It has good selectivity over both the hERG ion channel and closely related AGC kinase ROCK, and it shows pharmacodynamic and xenograft activity in vivo. It has potential in cancer therapy and is currently in phase 1 clinical trials. [1] AKT is a key node in the most frequently deregulated signaling network in human cancer. AZD5363, a novel pyrrolopyrimidine-derived compound, inhibited all AKT isoforms with a potency of 10 nmol/L or less and inhibited phosphorylation of AKT substrates in cells with a potency of approximately 0.3 to 0.8 μmol/L. AZD5363 monotherapy inhibited the proliferation of 41 of 182 solid and hematologic tumor cell lines with a potency of 3 μmol/L or less. Cell lines derived from breast cancers showed the highest frequency of sensitivity. There was a significant relationship between the presence of PIK3CA and/or PTEN mutations and sensitivity to AZD5363 and between RAS mutations and resistance. Oral dosing of AZD5363 to nude mice caused dose- and time-dependent reduction of PRAS40, GSK3β, and S6 phosphorylation in BT474c xenografts (PRAS40 phosphorylation EC(50) ~ 0.1 μmol/L total plasma exposure), reversible increases in blood glucose concentrations, and dose-dependent decreases in 2[18F]fluoro-2-deoxy-D-glucose ((18)F-FDG) uptake in U87-MG xenografts. Chronic oral dosing of AZD5363 caused dose-dependent growth inhibition of xenografts derived from various tumor types, including HER2(+) breast cancer models that are resistant to trastuzumab. AZD5363 also significantly enhanced the antitumor activity of docetaxel, lapatinib, and trastuzumab in breast cancer xenografts. It is concluded that AZD5363 is a potent inhibitor of AKT with pharmacodynamic activity in vivo, has potential to treat a range of solid and hematologic tumors as monotherapy or a combinatorial agent, and has potential for personalized medicine based on the genetic status of PIK3CA, PTEN, and RAS. AZD5363 is currently in phase I clinical trials. [2] In conclusion, AZD5363 is a potent inhibitor of AKT with a pharmacologic profile consistent with its mechanism of action in vitro and in vivo. Tumor types with PIK3CA mutation, PTEN mutation, or HER2 amplification, without coincident RAS mutation, show the highest frequency of response to AZD5363 in vitro; in such tumor types, stasis or regression is achievable by monotherapy dosing in vivo. AZD5363 also has potential to overcome resistance or increase sensitivity to HER2 inhibitors in breast cancer, and greatly sensitizes to docetaxel chemotherapy, resulting in tumor regression in vivo. AZD5363 is currently in phase I clinical trials. [2] |
| 分子式 |
C21H25CLN6O2
|
|---|---|
| 分子量 |
428.9
|
| 精确质量 |
428.172
|
| 元素分析 |
C, 58.81; H, 5.87; Cl, 8.27; N, 19.59; O, 7.46
|
| CAS号 |
1143532-51-7
|
| 相关CAS号 |
Capivasertib;1143532-39-1
|
| PubChem CID |
57750340
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.4±0.1 g/cm3
|
| 折射率 |
1.670
|
| LogP |
1.04
|
| tPSA |
120
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
580
|
| 定义原子立体中心数目 |
1
|
| SMILES |
N1(C2N=CN=C3NC=CC3=2)CCC(N)(C(N[C@@H](C2=CC=C(Cl)C=C2)CCO)=O)CC1
|
| InChi Key |
JDUBGYFRJFOXQC-QGZVFWFLSA-N
|
| InChi Code |
InChI=1S/C21H25ClN6O2/c22-15-3-1-14(2-4-15)17(6-12-29)27-20(30)21(23)7-10-28(11-8-21)19-16-5-9-24-18(16)25-13-26-19/h1-5,9,13,17,29H,6-8,10-12,23H2,(H,27,30)(H,24,25,26)/t17-/m1/s1
|
| 化学名 |
4-amino-N-[(1R)-1-(4-chlorophenyl)-3-hydroxypropyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamide
|
| 别名 |
(R)-Capivasertib; 1143532-51-7; 4-Piperidinecarboxamide, 4-amino-N-[(1R)-1-(4-chlorophenyl)-3-hydroxypropyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-; CHEMBL2325741; SCHEMBL391382; JDUBGYFRJFOXQC-QGZVFWFLSA-N; BDBM256836; BDBM50427349;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3315 mL | 11.6577 mL | 23.3155 mL | |
| 5 mM | 0.4663 mL | 2.3315 mL | 4.6631 mL | |
| 10 mM | 0.2332 mL | 1.1658 mL | 2.3315 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Targeted Therapy Directed by Genetic Testing in Treating Patients With Advanced Refractory Solid Tumors, Lymphomas, or Multiple Myeloma (The MATCH Screening Trial)
CTID: NCT02465060
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-11-18