| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 靶点 |
SSTR2; Somatostatin sst2 receptor
|
|---|---|
| 体外研究 (In Vitro) |
在表达人 sst2 受体的 CHO-K1 细胞中,CYN 154806 TFA 可防止 SRIF 诱导的细胞外酸化 (EAR) 增加 (pKB 7.92)。此外,在表达人 SST2 受体以及大鼠 SST2(a) 和大鼠 SST2(b) 受体(pKB 分别为 7.81、7.68 和 7.96)的 CHO-K1 细胞膜中,CYN 154806 TFA 抑制 SRIF 诱导的 [35S] 增加。 ]-GTPγS 结合[2]。
|
| 体内研究 (In Vivo) |
CYN 154806 TFA(0.1 mg/kg;腹腔注射;在 M4 KO 小鼠中注射卡巴胆碱 (CCh)(30 μg/kg)前 20 分钟)可显着且剂量依赖性地逆转 M4 但不是 M3 KO 小鼠对 CCh 的酸反应的减少[ 3]。
|
| 酶活实验 |
这些肽的功能特性已在放射性配体结合试验、SST2亚型与酵母信息素反应途径的功能偶联以及cAMP积累中确定。一种肽拮抗剂[Ac-4-NO2-Phe-c(D-Cys-Tyr-D-Trp-Lys-Thr-Cys)-D-Tyr-NH2](CYN 154806)显示出与天然激素(Ki=0.2 nM)相当的SST2结合亲和力,并逆转了生长抑素介导的对大鼠生长肌营养细胞GH4C1细胞、转染SST2和SST5亚型的细胞中cAMP积累的抑制,以及生长抑素刺激的表达SST2亚型的酵母细胞的生长。这类生长抑素拮抗剂是第一个被描述的,可用于测定生长抑素在体内和体外的不同功能[1]。
|
| 细胞实验 |
环八肽CYN-154806抑制了特异性[(125)I]-[Tyr(11)]-SRIF与表达人重组生长抑素(SRIF)sst(2)受体(pIC(50)8)的CHO-K1细胞膜的结合。58)或大鼠sst(2(a))和大鼠sst(2(b))受体(pIC(50)8.35和8。分别为10)。CYN-154806对其他人类生长抑素受体类型的亲和力至少低100倍(pIC(50)5。41-6.48). 在功能研究中,CYN-154806抑制了SRIF诱导的表达h-sst(2)受体(pK(B)7.92)的CHO-K1细胞细胞外酸化(EAR)的增加,但对UTP诱导的EAR增加没有影响。CYN-154806还阻断了SRIF诱导的CHO-K1细胞膜中[(35)S]-GTPγS结合的增加,该细胞膜表达h sst(2)受体以及大鼠sst(1(a))和大鼠sst(2(b))受体(分别为pK(b)7.81、7.68和7.96)。与此形成鲜明对比的是,在浓度高达10微M的h sst(5)受体上没有观察到阻断。在已知表达内源性SRIF受体的分离组织制剂中也研究了CYN-154806的拮抗活性。因此,CYN-154806阻断了SRIF,但没有阻断DAMGO诱导的大鼠离体输精管和豚鼠回肠神经源性收缩的抑制(分别为pK(B)7.79和7.49)。CYN-154806对SRIF-28诱导的豚鼠输精管神经源性收缩的抑制没有影响。结果表明,CYN-154806是一种高效的特异性和选择性SRIF sst(2)受体阻断药物。此外,sst(2)受体介导SRIF诱导的大鼠输精管和豚鼠回肠神经源性收缩的抑制,但不介导豚鼠输精管的抑制,后者被认为是由sst(5)受体介介导的[2]。
|
| 动物实验 |
CYN154806 were dissolved in dimethyl sulfoxide (DMSO) and diluted with distilled water to desired concentrations. Each agent was prepared immediately before use and administered as a single injection s.c. or i.p. in a volume of 1 ml per 100 g body weight.
In some cases CYN154806 (a somatostatin SST2 receptor antagonist: 0.1 mg/kg; Feniuk et al., 2000) was given i.p. 20 min before the administration of octreotide (20 μg/kg) in WT mice or the administration of CCh (30 μg/kg) in M4 KO mice. Control animals received saline or vehicle in place of the active agent. The doses of atropine, octreotide or CYN154806 were selected in order to induce the respective pharmacological actions according to the findings of previously published studies [3].
C57BL/6J mice of wild-type (WT) and M1-M5 KO were used. Under urethane anesthesia, acid secretion was measured in the stomach equipped with an acute fistula. CCh (30 μg/kg) was given subcutaneously (s.c.) to stimulate acid secretion. Atropine or octreotide (a somatostatin analog) was given s.c. 20 min before the administration of CCh. CYN154806 (a somatostatin SST2 receptor antagonist) was given i.p. 20 min before the administration of octreotide or CCh.[3] Results: CCh caused an increase of acid secretion in WT mice, and the effect was totally inhibited by prior administration of atropine. The effect of CCh was similarly observed in the animals lacking M1, M2 or M5 receptors but significantly decreased in M3 or M4 KO mice. CYN154806, the SST2 receptor antagonist, dose-dependently and significantly reversed the decreased acid response to CCh in M4 but not M3 KO mice. Octreotide, the somatostatin analog, inhibited the secretion of acid under CCh-stimulated conditions in WT mice. The immunohistochemical study showed the localization of M4 receptors on D cells in the stomach. Serum somatostatin levels in M4 KO mice were higher than WT mice under basal conditions, while those in WT mice were significantly decreased in response to CCh.[3] Conclusions: These results suggest that under cholinergic stimulation the acid secretion is directly mediated by M3 receptors and indirectly modified by M4 receptors. It is assumed that the activation of M4 receptors inhibits the release of somatostatin from D cells and minimizes the acid inhibitory effect of somatostatin through SST2 receptors, resulting in enhancement of the acid response mediated by M3 receptors on parietal cells.[3] |
| 参考文献 |
|
| 其他信息 |
Researchers also found that the suppressed acid response to CCh in M4 KO mice was significantly restored by the prior application of the somatostatin SST2 antagonist, CYN 154806. In addition, it was found that serum somatostatin levels were significantly increased in M4 KO mice under CCh-stimulated conditions. These results strongly support our hypothesis that the decrease of CCh-induced acid response in M4 KO mice is explained by the inhibitory effect of somatostatin mediated by SST2 receptors. It is therefore assumed that the activation of M4 receptors inhibits somatostatin release from D cells and negates the negative influence of this peptide on acid secretion, resulting in a potentiation of the acid response to CCh. Certainly, more studies are needed to clarify the regulatory mechanisms of somatostatin secretion from D cells.
Finally, it remains undefined whether M4 receptors are really expressed on D cells? We performed the immunostaining of the gastric mucosa with anti-somatostatin and anti-M4 receptor antibodies in WT mice. The histological observation showed that M4 receptors were co-expressed with somatostatin, indicating the expression of M4 receptors on D cells. We confirmed that M4 receptors were not observed in the stomachs of M4 KO mice. These results strongly suggest that CCh inhibits somatostatin release from D cells via the activation of M4 receptors. The present study was performed in mice anesthetized with urethane. Since this anesthetic is known to promote the secretion of somatostatin from D cells (Saito et al., 1979), the results obtained in this study might differ from those obtained under normal physiological conditions. However, since CYN 154806 by itself had no effect on basal acid secretion, it is assumed that the interpretation of the present results is not affected by urethane anesthesia.[3]
|
| 分子式 |
C58H69F3N12O16S2
|
|---|---|
| 分子量 |
1311.36448168755
|
| 精确质量 |
1310.4348
|
| CAS号 |
2828432-46-6
|
| 相关CAS号 |
CYN 154806;183658-72-2
|
| PubChem CID |
71312046
|
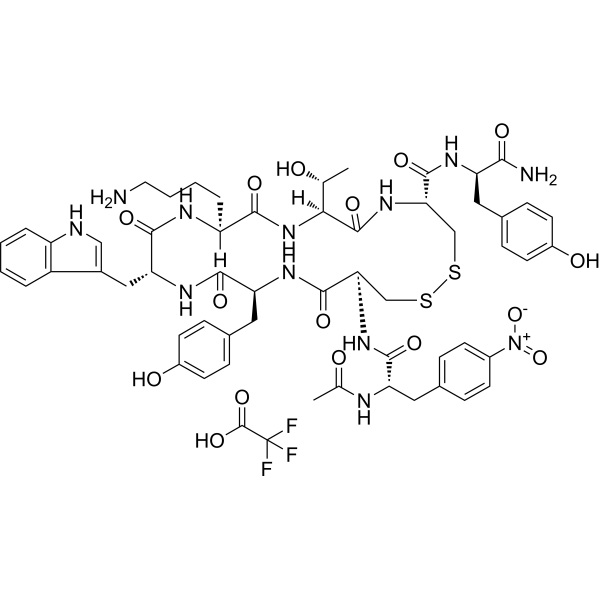
| 序列 |
XCYWKTCY; N-acetyl-4-nitro-L-phenylalanyl-D-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-threonyl-L-cysteinyl-D-tyrosinamide (2->7)-disulfide trifluoroacetic acid; Ac-Phe(4-NO2)-Cys-Tyr-Trp-Lys-Thr-Cys-Tyr-NH2 (Disulfide bridge: Cys2-Cys7)
|
| 短序列 |
Ac-F(4-NO2)CYWKTCY-NH2 (Disulfide bridge: Cys2-Cys7); Ac-Phe(4-NO2)-D-Cys(1)-Tyr-D-Trp-Lys-Thr-Cys(1)-D-Tyr-NH2.TFA
|
| 外观&性状 |
White to light yellow solid powder
|
| LogP |
4.8
|
| tPSA |
512Ų
|
| 氢键供体(HBD)数目 |
15
|
| 氢键受体(HBA)数目 |
22
|
| 可旋转键数目(RBC) |
19
|
| 重原子数目 |
91
|
| 分子复杂度/Complexity |
2330
|
| 定义原子立体中心数目 |
9
|
| SMILES |
C(F)(F)(F)C(=O)O.C([C@@H]1C(N[C@]([H])(C(N[C@]([H])(C(N[C@H](C(=O)N[C@@H](C(=O)N)CC2C=CC(O)=CC=2)CSSC[C@H](NC(=O)[C@@H](NC(=O)C)CC2C=CC(N(=O)=O)=CC=2)C(=O)N[C@@H](CC2C=CC(O)=CC=2)C(=O)N1)=O)[C@H](O)C)=O)CCCCN)=O)C1=CNC2=CC=CC=C12
|
| InChi Key |
FYVCFCQFOBEWIO-GVGJFGMUSA-N
|
| InChi Code |
InChI=1S/C56H68N12O14S2.C2HF3O2/c1-30(69)48-56(80)66-47(54(78)62-42(49(58)73)23-33-12-18-37(71)19-13-33)29-84-83-28-46(65-51(75)43(60-31(2)70)24-32-10-16-36(17-11-32)68(81)82)55(79)63-44(25-34-14-20-38(72)21-15-34)52(76)64-45(26-35-27-59-40-8-4-3-7-39(35)40)53(77)61-41(50(74)67-48)9-5-6-22-57;3-2(4,5)1(6)7/h3-4,7-8,10-21,27,30,41-48,59,69,71-72H,5-6,9,22-26,28-29,57H2,1-2H3,(H2,58,73)(H,60,70)(H,61,77)(H,62,78)(H,63,79)(H,64,76)(H,65,75)(H,66,80)(H,67,74);(H,6,7)/t30-,41+,42-,43+,44+,45-,46-,47+,48+;/m1./s1
|
| 化学名 |
(4R,7S,10S,13R,16S,19S)-19-[[(2S)-2-acetamido-3-(4-nitrophenyl)propanoyl]amino]-10-(4-aminobutyl)-N-[(2R)-1-amino-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]-7-[(1R)-1-hydroxyethyl]-16-[(4-hydroxyphenyl)methyl]-13-(1H-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carboxamide;2,2,2-trifluoroacetic acid
|
| 别名 |
YN 154806 (TFA); CYN 154806 TFA; CYN 154806 trifluoroacetate salt; FYVCFCQFOBEWIO-GVGJFGMUSA-N; 2828432-46-6;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O: 25 mg/mL (19.06 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.7626 mL | 3.8128 mL | 7.6256 mL | |
| 5 mM | 0.1525 mL | 0.7626 mL | 1.5251 mL | |
| 10 mM | 0.0763 mL | 0.3813 mL | 0.7626 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。