| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 1g |
|
||
| Other Sizes |
|

| 靶点 |
Topoisomerase
|
|---|---|
| 体外研究 (In Vitro) |
Exatecan作为高效TOP1抑制剂的生化和结构基础[1]
DXd是通过2-羟基乙酰基(COCH2OH)基团对F环上的NH2进行化学修饰而从艾替康衍生而来的(补充图S1A)。在一组癌症细胞系中,Exatecan的体外细胞毒性平均是DXd的10至20倍,具有亚纳米级IC50(补充图S1B;补充表S1)。 基于细胞的TOP1抑制试验和结构建模提供了艾替康更高细胞毒性效力的机制。通过改进的DNA加合物回收快速方法(RADAR)测定法检测艾替康与DXd和SN-38相比的DNA捕获TOP1(TOP1ccs)水平(21)。Exatecan是最有效的药物,其诱导TOP1ccs的浓度低于DXd和SN-38(图1A;补充图S1C)。捕获的DNA TOP1通常从DNA中去除并降解。为了测量TOP1降解率,细胞用艾替康或DXd/SN-38处理2小时,然后在没有药物的情况下生长30分钟,以逆转TOP1cs和TOP1降解。Exatecan以剂量依赖的方式诱导TOP1降解,比DXd和SN-38更有效(图1B)。 Exatecan对MDR机制的敏感性低于DXd和SN38[1] 在Caco-2细胞中测定了艾考替康、DXd和SN-38的MDR转运蛋白ABCG2和P-gp介导的外排(补充图S1E)。DXd显示出比没有或有ABCG2抑制剂新生霉素、P-gp抑制剂维拉帕米或双抑制剂GF120918的艾替康高一个数量级的外排率(图1D)。有趣的是,SN-38的流出率高于艾替康,但低于DXd。艾替康和DXd细胞内积累的免疫荧光(IF)检测表明,在ABCG2高表达细胞系NCI-H460中,DXd的积累低于艾替康(图1E;补充图S1F-1J)。 与MDR底物实验结果一致,DXd和艾替康之间的细胞毒性效力差异与内源性ABCG2/P-gp表达(mRNA和蛋白质;补充图S2A-S2C)相关。一般来说,在ABCG2或P-gp表达较高的细胞中,DXd/exatecan的IC50比值较高(图1F;补充图S2D和S2E)。ABCG2或P-gp的抑制提高了DXd/SN-38的细胞毒性,但对艾替康的影响要小得多(图1G)。即使DXd/SN-38抑制剂的IC50有所提高,艾替康仍然更有效(补充图S2F和S2G)。总的来说,exatecan对MDR基因的敏感性低于DXd/SN38,这可能在癌症细胞中赋予更高的细胞毒性以及更普遍的耐药性机制。 |
| 体内研究 (In Vivo) |
使用DNA拓扑异构酶I抑制剂DXd/SN-38的抗体-细菌偶联物(ADC)已经改变了癌症的治疗,但需要更有效的ADC来克服耐药性。我们设计了一种ADC类,使用一种新型的自焚T部分,用于艾替康的无痕结合和释放,艾替康是一种更有效的拓扑异构酶I抑制剂,对多药耐药性(MDR)的敏感性较低。以增强的治疗指数、更高的稳定性和改善的肿瘤内药效学反应为特征,靶向HER2、HER3和TROP2的抗体-T部分艾考替康偶联物克服了低靶表达、大和MDR+肿瘤中等效DXd/SN-38 ADC的内在或治疗耐药性。T部分脱羧酶ADC在患者来源的异种移植物和类器官模型中显示出持久的抗肿瘤活性,这些模型代表了未满足的临床需求,包括EGFR ex19del/T790M/C797S三突变肺癌和BRAF/KRAS-TP53双突变癌症,并显示出与PARP/ATR抑制剂和抗PD-1治疗的协同作用。T部分艾考替康ADC类在非人灵长类动物中的高耐受性支持其将反应患者群体和肿瘤类型扩展到当前ADC之外的潜力。
意义:结合新型自焚部分和拓扑异构酶I抑制剂exatecan作为有效载荷的ADC在低靶表达和耐DXd/SN-38 ADC的MDR+肿瘤中显示出深度和持久的反应,而不会增加毒性。这种新型ADC有可能使现有选择之外的更多患者受益。见Gupta等人的相关评论,第817页。这篇文章在《in This Issue》特刊第799[1]页中突出显示。
|
| 细胞实验 |
MTX-1000/DS-8201a在血浆中的体外稳定性[1]
在小鼠、大鼠、猴子和人血浆中评估了37°C下10 mg/mL浓度下MTX-1000/DS-8201a在21天内艾替康/DXd的释放速率。 ELISA[1] 对于结合试验,免疫板在包被缓冲液中用2.5 mg/mL His标记的HER2-ECD蛋白包被,并在4°C下保持过夜。洗涤后,将板封闭,并将每种连续稀释的物质加入孔中。在37°C下孵育1.5小时后,清洗平板,并在37°C下用HRP偶联的抗人IgG二抗孵育1小时。洗涤后,加入TMB溶液,用微孔板读数器测量每个孔中的A450。为了检测磷酸化Akt(pAkt),将SK-BR-3细胞在96孔板中预孵育4天,然后与每种物质孵育24小时。孵育后,根据制造商的说明,裂解细胞,使用PathScan Phospho-Akt1(Ser473)夹心ELISA试剂盒和PathScan total-Akt1夹心ELISA试剂袋检测细胞间pAkt和总Akt。通过将处理过的归一化pAkt值除以未处理的归一化pAk值来计算每个样品孔的相对pAkt。 免疫印迹[1] 用每种物质处理KPL-4细胞。24、48或72小时后,收获细胞并用含有Halt蛋白酶和磷酸酶抑制剂混合物的M-PER裂解缓冲液裂解。通过SDS-PAGE对样品进行装载和分离,并将其印迹到聚偏二氟乙烯膜上。用抗磷酸化Chk1(Ser345;133D3)兔单克隆抗体、抗Chk1(2G1D5)小鼠单克隆抗体、反切割PARP(Asp214)抗体、抗β-肌动蛋白(8H10D10)小鼠mAb、抗磷酸化组蛋白H2A将膜封闭并探测过夜。X(Ser139)抗体和抗组蛋白H2A。4°C下的X抗体(Abcam)。然后,用SNAP皮内洗涤膜并用荧光标记的二抗孵育10分钟。使用Odyssey成像系统检测荧光信号。 |
| 动物实验 |
Cell Line and PDX Studies [1]
All tumor-bearing models were established in female BALB/c nude mice that were purchased from the Shanghai Family Planning Research Institute. All in vivo studies were performed in accordance with the local guidelines of the Institutional Animal Care and Use Committee of Multitude Therapeutics and with the approval of the committee. Briefly, 4- to 6-week-old mice were housed together in sterilized cages and maintained under required pathogen-free conditions. Each cell suspension or tumor fragment was inoculated subcutaneously into female nude mice. When the tumor had grown to an appropriate volume, the tumor-bearing mice were randomized into treatment and control groups based on the tumor volumes, and dosing was started. Antibodies and ADCs were administered intravenously to the mice. Small-molecule drugs were administered orally. Tumor volume defined as 1/2 × length × width2 was measured twice (3–4 days) a week. TGI (%) was calculated as follows: TGI (%) = [1 – (mean of treatment group tumor volume on evaluation day)/(mean of control group tumor volume on evaluation day)] × 100. The mice were euthanized with CO2 gas when they reached endpoints (tumor volume exceeding 3,000 mm3, >10% reduction of body weight, or clinical signs indicating that mice should be euthanized for ethical reasons). Syngeneic Model [1] Female BALB/c mice, weighing approximately 18 to 20 g, were maintained with 5 mice per cage in an individually ventilated cage in a specific pathogen–free animal facility with autoclaved cages. After 9 days of acclimation, animals were subcutaneously inoculated with CT26-hHER2 cells (3 × 105 cells/0.1 mL/mouse) on the right flank for tumor development. Ten days after inoculation, 36 mice with tumor sizes ranging from 100 to 200 mm3 (average tumor size 178 mm3) were enrolled into the efficacy study and randomized into six therapy groups, and the treatments were initiated on the randomization day (defined as D0). MTX-1000, DS-8201a (10 mg/kg), and anti–PD-1 antibody (5 mg/kg) were administered intravenously at a volume of 10 mL/kg to mice. As a control, ABS buffer (10 mmol/L acetate buffer, 5% sorbitol, pH 5.5) was administered at the same volume as the drugs. MTX-1000 and DS-8201a were administered on days 0 and 7. Anti–PD-1 antibody was administered on days 0, 3, 7, and 10. For flow-cytometric analysis of T cells, dendritic cells, and tumor cells, when the average volume of tumors reached approximately 250 to 400 mm3 (8 days after tumor inoculation), the mice were treated with vehicle or DS-8201a (10 mg/kg, once, intravenously; day 0). The mice were euthanized with CO2 asphyxiation on day 8, and tumors were cut into small pieces and dissociated with the Tumor Dissociation Kit by the gentleMACS Octo Dissociator with Heaters. The resultant single cells were blocked with Mouse BD Fc Block reagent and stained with antibodies against mouse CD3, CD4, CD8, CD11c, CD45, CD86, granzyme B, MHC class I, MHC class II, and PD-L1 and human HER2. Tissue Multiplex IHC [1] Sections from formalin-fixed, paraffin-embedded tumor tissue blocks were stained using Opal multiplex according to the manufacturer's protocol for DAPI, CD45, CD4, and CD8. Slide scanning was performed on the Vectra 3.0 instrument. All the images were analyzed with the HALOTM Image Analysis platform. The whole section was analyzed, and the big necrosis area and stroma area were excluded. Single-positive cells were counted separately. Images were spectrally unmixed, evaluated for staining intensities and morphology, tissue segmented based on tissue markers, cell segmented based on nuclear and membrane markers, and phenotypically scored. PK of MTX-1000/DS-8201a in Rat/Mouse [1] Concentrations of ADC and the total antibody in plasma were determined with a validated ligand-binding assay; the lower limit of quantitation was 0.02 μg/mL. Briefly, immunoplates were coated with 1 μg/mL Human HER2 Protein, His Tag in coating buffer and kept overnight at 4°C. After washing, the plates were blocked, and each serially diluted sample was added to the wells. After incubation for 1 to 2 hours at 37°C, the plates were washed and incubated with HRP-conjugated anti-human IgG Fc secondary antibody for total antibody measurement or a biotin-labeled anti-exatecan/DXd antibody for ADC measurement. After reaction at 37°C for 1 to 2 hours, TMB solution was added directly or after incubation with Streptavidin Protein, HRP for 40 to 60 minutes at 37°C. A450 in each well was measured with a microplate reader. Concentrations of payload exatecan/DXd in plasma were determined with a validated LC/MS-MS method; the lower limit of quantitation was 0.05 ng/mL. MTX-1000/DS-8201a was intravenously administered at 4.0 mg/kg to rats. Plasma concentrations of ADC, total antibody, and DXd were measured up to 21 days after dose. |
| 药代性质 (ADME/PK) |
T Moiety Conjugation Translates Exatecan into a Superior ADC: Physicochemical and Pharmacologic Profile, Stability, and Toxicity [1]
T Moiety Design: Hydrophilic Modulation of the PABC Spacer for Traceless Conjugation [1] To enable a traceless conjugation and release of exatecan, we used a dipeptide VA linker and a modified self-immolative spacer p-amino benzyl (pAB) called T moiety (Fig. 2A; S1 in Supplementary Fig. S4A). VA was selected because it creates more hydrophilic high-DAR ADCs compared with other peptide linkers VC or Gly–Gly–Phe–Gly (GGFG; S0 in Supplementary Fig. S4A; refs. 29–30). Direct conjugation of HER2-targeting Tras and exatecan using the unmodified peptide linker VA/VC/GGFG caused high aggregation (Supplementary Fig. S4B). Integrating T moiety with a PEG group (T900; S3 in Supplementary Fig. S4A) significantly reduced aggregation to an acceptable level of 2%. However, modification of MC with the same PEG (called M moiety, M900; S2 in Supplementary Fig. S4A) led to a high aggregation of >50% (Supplementary Fig. S4B), highlighting the importance of the judicious selection of modification position. Considering the potential lethal adverse reactions caused by PEG-based biological drugs (31), we decided to use polysarcosine (pSAR) due to its higher solubility and biocompatibility (Supplementary Fig. S4C; ref. 32). Polysarcosine with 10 units (pSAR10) modified pAB (T1000) resulted in homogeneous ADC with a negligible level of aggregation (Supplementary Fig. S4B). Remarkably, an intermediate structure T800 with m-methylaminomethyl modification of pAB for attaching pSAR10 produced homogeneous ADC of high DAR without aggregation. In comparison, the attachment of the same m-methylaminomethyl group to the VA linker (VK-pABC, M800, CLogP = 4.98) caused 10% aggregation with exatecan even when T800 (CLogP = 4.73) and M800 are considered to be chemically equivalent, supporting pAB selection as the optimal modification site. The conjugation yield for T moiety ADCs was >90%. Replacing the VA linker with GGFG in T1000 also yielded homogeneous ADC without aggregation (T1001; Supplementary Fig. S4B). Tras–T1000–Exatecan (MTX-1000) Shows a Superior Physicochemical Profile [1] To compare the physicochemical parameters of Tras–T1000–exatecan (MTX-1000) with those of DS-8201a, we used either purchased DS-8201a or internally manufactured Tras–GGFG–DXd with closely matched physicochemical profiles (Supplementary Fig. S5A and S5B), in vivo potency (Supplementary Fig. S5C–S5E), and pharmacokinetics (PK) in mice (Supplementary Fig. S5F). The attachment of exatecan to the HER2 antibody did not affect HER2 antibody binding to target by flow cytometry or ELISA, similar to Tras–GGFG–DXd (Supplementary Fig. S6A and S6B). Tras–exatecan conjugates using T800 or T900 also resulted in more hydrophilic ADCs than Tras–GGFG–DXd (Supplementary Fig. S6C and S6D). Both DAR8 and DAR4 ADCs were readily generated (Supplementary Fig. S6E). Tras–T moiety–exatecan ADCs (Tras–T800–exatecan/MTX-800 and Tras–T1000–exatecan/MTX-1000, both DAR8) demonstrated similar or better stability than Tras–GGFG–DXd. This was measured by aggregation formed for an extended incubation time at 37°C and under repeat freeze–thaw cycles (Supplementary Fig. S6F). T moiety–exatecan ADCs also showed better thermostability and photostability than DS-8201a (Supplementary Fig. S6G and S6H). Notably, VA and pSAR ADC (T1000) showed better stability and less aggregation than other linker (GGFG) and modification (PEG; T900 or T1001; Supplementary Fig. S6C, S6D, S6F, and S6G), highlighting the impact of linker and conjugation chemistry choice on the physicochemical function of an ADC. |
| 毒性/毒理 (Toxicokinetics/TK) |
Exatecan Mesylate Is Well Tolerated in Rats[1]
We conducted a 4-week (days 1, 8, 15, 22, and 29) intermittent intravenous dose toxicity study of exatecan mesylate in rats (6 animals/group) with a 4-week recovery period (3 of the 6 animals). Exatecan mesylate was well tolerated in rats at doses up to 10 mg/kg (calculated as exatecan-free base). At 30 mg/kg, exatecan mesylate resulted in decreased animal body weight, morbidity (decreased food consumption, abnormal clinical signs), and/or mortality (occurred 5 or 6 days after dose). The dose-dependent transient suppression of body weight gain (Supplementary Fig. S3A), decrease of blood cell counts (Supplementary Fig. S3B–S3E), and increase of key serum enzyme levels (Supplementary Fig. S3F and S3G) were observed in 3 mg/kg and 10 mg/kg groups but largely reversible by the end of the 4-week recovery period (Supplementary Table S2), suggesting that exatecan was similarly tolerated to DXd or SN-38. |
| 参考文献 |
|
| 其他信息 |
T moiety–exatecan ADCs maintained a higher potency without increasing toxicity with HNSTDs in nonhuman primate studies at a level that was similar to or higher than that of other TOP1 inhibitor ADCs. Notably, side effects from the administration of T moiety–exatecan ADC were often more prominently observed in the GI tract (diarrhea) and the hematologic system (reticulocyte reduction), though symptoms in both were fully reversible. The hematologic and digestive organ toxicities for T moiety–exatecan ADCs mirrored those of exatecan as a single agent in human trials. However, T moiety–exatecan ADC achieved more favorable safety signals in the GI tract and minimal myelotoxicity than exatecan, possibly reflecting the ADC design's superior physicochemical features. Improved stability, longer ADC half-life, lower free payload release, and the reduced myelotoxicity potential of ADC in comparison with free payload may all contribute to the favorable toxicity profile of T moiety–exatecan ADC. Interstitial lung disease occurred in just over 10% of DXd-based ADC–treated patients during clinical trials. Although we did not observe any structural damage or signs of inflammation in the lungs of animals dosed with T moiety–exatecan ADCs, it remains uncertain whether prolonged treatment will yield additional pulmonary toxicity. Nevertheless, it is conceivable that T moiety–exatecan ADCs could have different target-independent tissue distributions than DXd-based ADCs, resulting in the manifestation of diverse symptoms.
For tumors less responsive or resistant to TOP1 inhibitors, payload classes with different mechanisms may be necessary. T moiety is a modular structure compatible with diverse linker chemistry and is amenable to building ADCs with dual payloads. Taken together, T moiety–exatecan ADCs have the potential to address patient needs unfulfilled by current ADCs, whereas the versatility and scalability of T moiety can facilitate the introduction of payloads of completely different MOAs to meet the continuous challenges of drug resistance.[1] |
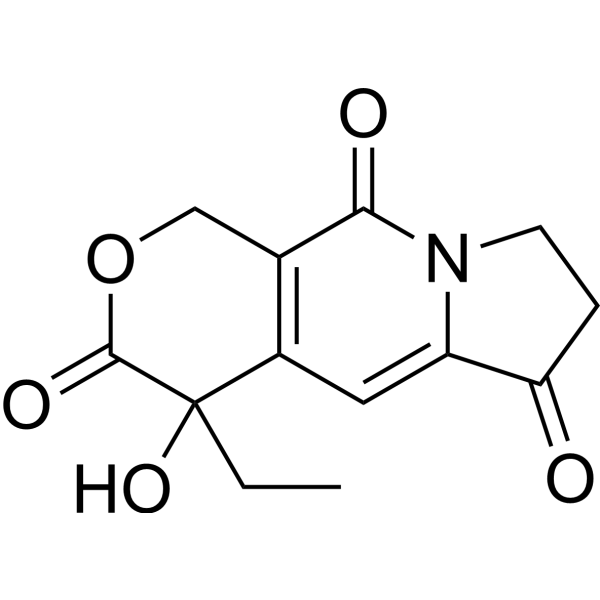
| 分子式 |
C13H13NO5
|
|---|---|
| 分子量 |
263.25
|
| 精确质量 |
263.079
|
| CAS号 |
102978-40-5
|
| 相关CAS号 |
Exatecan Intermediate 1;110351-94-5;(R)-Exatecan Intermediate 1;110351-91-2
|
| PubChem CID |
359849
|
| 外观&性状 |
White to light yellow solid powder
|
| LogP |
0.089
|
| tPSA |
85.6
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
19
|
| 分子复杂度/Complexity |
574
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
IGKWOGMVAOYVSJ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C13H13NO5/c1-2-13(18)8-5-9-10(15)3-4-14(9)11(16)7(8)6-19-12(13)17/h5,18H,2-4,6H2,1H3
|
| 化学名 |
4-ethyl-4-hydroxy-7,8-dihydro-1H-pyrano[3,4-f]indolizine-3,6,10-trione
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7987 mL | 18.9934 mL | 37.9867 mL | |
| 5 mM | 0.7597 mL | 3.7987 mL | 7.5973 mL | |
| 10 mM | 0.3799 mL | 1.8993 mL | 3.7987 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。