| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Ca2+/Calmodulin-dependent protein kinase kinase(CaM-KK); CaM-KKα (Ki = 80 ng/mL); CaM-KKβ (Ki = 15 ng/mL)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
STO-609 抑制重组 CaM-KKα 和 CaM-KKβ 亚型的自磷酸化和活性,Ki 值分别为 80 和 15 ng/mL。 STO-609 对 CaM-KII 的 IC50 值为 10 μg/mL,对 CaM-KK 具有高度选择性,对下游 CaM 激酶(CaM-KI 和 -IV)没有明显影响。野生型酶和组成型活性 CaM-KKα 均被 STO-609 抑制。 STO-609 以剂量依赖性方式抑制转染 HeLa 细胞中 Ca2+ 诱导的 CaM-KIV 激活。在1μg/mL浓度(80%抑制率)下,STO-609显着降低SH-SY5Y神经母细胞瘤细胞中CaM-KK的内源活性[1]。
|
||
| 体内研究 (In Vivo) |
体内施用 STO-609 会导致成骨细胞增加和破骨细胞减少,从而显着保护成年小鼠免受卵巢切除 (OVX) 诱导的骨质疏松症的影响。体内 ICV 施用 STO-609 不会影响对神经血糖减少症的反调节反应和血糖减少症诱导的 AMPK 激活。
|
||
| 酶活实验 |
钙调素KK活性的体外测定[1]
纯化的重组CaM KK(CaM KKα,0.28μg/ml;CaM KKβ,0.52μg/ml;组成型活性CaM KK,0.3μg/ml)与10μg GST CaM KI-(1–293)-K49E在30°C下在含有50 mm HEPES(pH 7.5)、10 mm Mg(Ac)2、1 mm DTT、不同浓度[γ-32P]ATP(650–6500 cpm/pmol)和不同浓度STO-609的溶液(25μl)中孵育5分钟。在1 mm EGTA(用于组成型活性CaM KK)或1 mm CaCl2、2μm CaM的存在下,在终浓度为4%的Me2SO中为10μg/ml。通过加入[γ-32P]ATP引发反应,将等分试样(15μl)点样到磷酸纤维素纸上,然后用75mm磷酸洗涤数次,终止反应。通过过滤器的液体闪烁计数测定磷酸盐掺入GST-CaM-KI-(1-293)-K49E的情况。基于最近描述的时间过程实验,选择5分钟的反应来测定CaM KK活性。计算出在没有STO-609的情况下,CaM KKα、CaM KKβ和组成型活性CaM KK的比活性分别为723±7μmol/min/mg、338±18μmol/min/mmg和927±40μmol/minmg/mg。 钙调素KKα和-β的自磷酸化[1] 在30°C下,在含有50 mm HEPES(pH 7.5)、10 mm Mg(Ac)2、1 mm DTT、50μm[γ-32P]ATP(6500 cpm/pmol)的溶液(25μl)中,在1 mm EGTA(用于CaM KKα和CaM KKβ)或1 mmCaCl2、2μm CaM(用于CaM KKα)的情况下,在30℃下对纯化的重组钙调素KKα和-β(0.8μg)进行5分钟的测定,其中含有不同浓度的STO-609(在Me2SO中为0-10μg/ml,终浓度为4%)。这是因为。通过加入[γ-32P]ATP引发反应,并通过加入SDS-PAGE样品缓冲液终止反应。样品经过SDS-10%PAGE,然后进行放射自显影。通过x射线胶片的密度扫描估计32P掺入CaM KK。 CaM KI、-II、-IV和MLCK活性的体外检测[1] 在含有50 mm HEPES(pH 7.5)、10 mm Mg(Ac)2、1 mm DTT、50μm[γ-32P]ATP(4500 cpm/pmol)和不同浓度STO-609(在Me2SO中为0-10μg/ml,最终浓度为4%)。如上所述,通过磷酸纤维素过滤法测量蛋白激酶活性。计算出在没有STO-609的情况下,CaM KI、CaM KII、CaM KITV和MLCK的比活性分别为24±1μmol/min/mg、122±3μmol/min/mmg、48±1μmol/L和178±6μmol/L。 PKA、PKC和p42 MAP激酶活性的体外检测[1] 在含有50 mm HEPES(pH 7.5)、10 mm mg(Ac)2、1 mm DTT、50μm[γ-32P]ATP(4500 cpm/pmol)和不同浓度STO-609(0在不存在(PKA和p42 MAP激酶)或存在1 mm CaCl2、0.4 mg/ml磷脂酰丝氨酸和0.1 mg/ml牛血清白蛋白的情况下,在终浓度为4%的Me2SO中为10μg/ml。如上所述,通过磷酸纤维素过滤法测量蛋白激酶活性。在没有STO-609的情况下,PKA、PKC和p42 MAP激酶的比活性分别为22±1μmol/min/mg、7.522±0.062 mmol/min/mg和181±3μmol/minmg/mg。基于每种酶的滴定实验结果,在线性条件下测量蛋白激酶活性。 激酶测定[1] AMPK肽,包括AMPK磷酸化位点周围的序列(167GEFLRTSCGSP177),在RIKEN脑科学研究所(BSI)研究资源中心(RRC)的生物材料分析支持单元合成。在30°C下,在有或没有500μm AMPK肽的情况下,在含有50 mm HEPES(pH 7.5)、300 mm NaCl、1 mm DTT、10 mm MgCl2、400μm ATP和10%甘油的反应溶液(20μl)中孵育适量的纯化CaMKKβKD和全长CaMKK?,有或没有0.5μmSTO-609。对于全长CaMKKβ,向反应溶液中加入5μm钙调素和1mm CaCl2。ATP消耗量通过使用激酶-GloTM Max发光激酶测定(Promega)试剂盒测定,该试剂盒定量反应溶液中的ATP量。10分钟后,使用FusionTM通用微孔板分析仪记录辉光型发光 |
||
| 细胞实验 |
HA-CaM-KIV[1]的瞬时表达和免疫沉淀
HeLa细胞保存在含有10%胎牛血清的Dulbecco改良Eagle培养基中。转染前12小时,细胞在6-cm培养皿中传代培养。然后将细胞转移到无血清培养基中,用3μg pME18s质粒DNA或3μg HA(血凝素标记)-CaM-KIV和20μg LipofectAMINE试剂在2.5ml培养基中的混合物处理。孵育20小时后,细胞在无血清培养基中进一步培养6小时,无论是否存在不同浓度的STO-609(0.01-10μg/ml的Me2SO溶液,终浓度为0.5%),然后用或不用1μm离子霉素处理5分钟。通过加入1ml裂解缓冲液(150mm NaCl、20mm Tris-HCl(pH 7.5)、2mm EDTA、2mm EGTA、1%Nonidet P-40、10%甘油、0.2mm苯甲基磺酰氟、10mg/l亮肽、10mg/l胰蛋白酶抑制剂和1μm微囊藻毒素终止刺激。LR)并将细胞在冰上裂解30分钟。收集细胞提取物,在15000×g下离心15分钟,上清液在4°C下用40μl g-Sepharose蛋白(50%浆液)预滤2小时,上清液与4μg抗HA抗体混合3小时。然后将40μl g-Sepharose蛋白质涂在提取物上并孵育过夜。如上所述,用1ml裂解缓冲液洗涤免疫沉淀的树脂三次,然后用1ml激酶缓冲液(50mm HEPES(pH 7.5)、10mmMg(Ac)2、1mm DTT、1mm EGTA和1μm微囊藻毒素LR)洗涤。如上所述,在1mm EGTA的存在下,使用syntide-2作为底物,对具有免疫沉淀的HA-CaM-KIV的G-Sepharose蛋白进行蛋白激酶测定(50μl反应体积)。为了估算免疫沉淀的HA-CaM-KIV的量,将SDS-PAGE样品缓冲液(50μl)加入免疫沉淀的样品中,然后在95°C下加热10分钟。离心后,将10μl样品进行SDS-10%PAGE,然后使用抗CaM-KIV抗体(1:2000)进行蛋白质印迹。
重组腺病毒感染SH-SY5Y神经母细胞瘤细胞后Ca2+/CaM非依赖性CaM-KIV的表达[1] 如下构建携带编码Ca2+/CaM非依赖性CaM KIV(305HMDT-DEDD)、激酶缺陷突变体(305HMDD-DEDD,K71E)或组成型活性CaM KK-(1-434)(3)的cDNA的重组腺病毒。简而言之,将pME18s质粒中的CaM KIV突变体和组成型活性CaM KK cDNA消化、钝端,然后连接到pShuttle(CLONTECH)中。根据制造商的方案,使用腺-X表达系统(CLONTECH)从HEK293细胞中获得重组病毒。对于病毒感染,将6孔培养板中的融合SY5Y细胞在37°C下以10个噬斑形成单位/细胞的多重感染方式感染病毒1小时。感染后,吸出病毒,并将细胞在含有10%胎牛血清的RPMI培养基中进一步培养12小时。然后,在不存在或存在不同浓度的STO-609(终浓度为0.5%的Me2SO中0.01-10μg/ml)的情况下,将细胞血清饥饿6小时。在不存在或存在不同浓度的STO-609的情况下,用1μm离子霉素刺激细胞10分钟(或不进行离子霉素处理),然后裂解,提取物进行SDS-7.5%PAGE,然后使用抗CaM KIV抗体进行蛋白质印迹。通过x射线胶片的密度扫描测量免疫反应带的强度。 |
||
| 动物实验 |
|
||
| 参考文献 | |||
| 其他信息 |
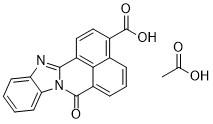
LSM-3164 is a naphthoic acid.
STO-609, a selective inhibitor of Ca(2+)/calmodulin-dependent protein kinase kinase (CaM-KK) was synthesized, and its inhibitory properties were investigated both in vitro and in vivo. STO-609 inhibits the activities of recombinant CaM-KK alpha and CaM-KK beta isoforms, with K(i) values of 80 and 15 ng/ml, respectively, and also inhibits their autophosphorylation activities. Comparison of the inhibitory potency of the compound against various protein kinases revealed that STO-609 is highly selective for CaM-KK without any significant effect on the downstream CaM kinases (CaM-KI and -IV), and the IC(50) value of the compound against CaM-KII is approximately 10 microg/ml. STO-609 inhibits constitutively active CaM-KK alpha (glutathione S-transferase (GST)-CaM-KK-(84-434)) as well as the wild-type enzyme. Kinetic analysis indicates that the compound is a competitive inhibitor of ATP. In transfected HeLa cells, STO-609 suppresses the Ca(2+)-induced activation of CaM-KIV in a dose-dependent manner. In agreement with this observation, the inhibitor significantly reduces the endogenous activity of CaM-KK in SH-SY5Y neuroblastoma cells at a concentration of 1 microg/ml (approximately 80% inhibitory rate). Taken together, these results indicate that STO-609 is a selective and cell-permeable inhibitor of CaM-KK and that it may be a useful tool for evaluating the physiological significance of the CaM-KK-mediated pathway in vivo as well as in vitro.[1] In summary, we have recently developed a potent and relatively selective inhibitor of CaM-KK, STO-609, which can permeate cells and which is a competitive inhibitor of ATP. Recent studies demonstrate that CaM-KK is a regulatory protein kinase for CaM-KI and CaM-KIVin vitro and in transfected cells; however, this property has not been demonstrated directly in vivo. We have shown in this report that STO-609 suppresses CaM-KK activity resulting in the inhibition of downstream CaM-KIV activity in intact cells, although it cannot inhibit downstream CaM kinase activities in vitro. Thus STO-609 could be a useful tool for evaluating the regulatory roles of CaM-KK for various physiological functions of the CaM kinase cascade such as the regulation of gene expression mediated by the CaM-KIV pathway. Furthermore, STO-609 could be used to distinguish between the functions of the two CaM-KK isoforms because the sensitivity of CaM-KKβ to the compound is ∼5-fold higher than that of the α isoform. The mechanism of differential sensitivity of CaM-KK isoforms to STO-609 is not clear, but it is not likely due to their differences in affinity for ATP because the apparent Km values of CaM-KKα and CaM-KKβ for ATP are indistinguishable (∼33 μm, Fig. 3B). Furthermore, physiological function(s) controlled by the CaM-KK/CaM-KI cascade have not been well studied, in contrast to what is known for CaM-KIV, and this question may be addressed with the use of STO-609. [1] Ca(2+)/calmodulin (CaM)-dependent protein kinase (CaMK) kinase (CaMKK) is a member of the CaMK cascade that mediates the response to intracellular Ca(2+) elevation. CaMKK phosphorylates and activates CaMKI and CaMKIV, which directly activate transcription factors. In this study, we determined the 2.4 Å crystal structure of the catalytic kinase domain of the human CaMKKβ isoform complexed with its selective inhibitor, STO-609. The structure revealed that CaMKKβ lacks the αD helix and that the equivalent region displays a hydrophobic molecular surface, which may reflect its unique substrate recognition and autoinhibition. Although CaMKKβ lacks the activation loop phosphorylation site, the activation loop is folded in an active-state conformation, which is stabilized by a number of interactions between amino acid residues conserved among the CaMKK isoforms. An in vitro analysis of the kinase activity confirmed the intrinsic activity of the CaMKKβ kinase domain. Structure and sequence analyses of the STO-609-binding site revealed amino acid replacements that may affect the inhibitor binding. Indeed, mutagenesis demonstrated that the CaMKKβ residue Pro(274), which replaces the conserved acidic residue of other protein kinases, is an important determinant for the selective inhibition by STO-609. Therefore, the present structure provides a molecular basis for clarifying the known biochemical properties of CaMKKβ and for designing novel inhibitors targeting CaMKKβ and the related protein kinases. [2] In conclusion, this study has demonstrated the unique structural properties of the CaMKKβ KD, which provide a molecular basis for understanding the known biochemical properties of CaMKKβ and the distinct STO-609 sensitivity between the CaMKKα and CaMKKβ isoforms. Our structure of the CaMKKβ·STO-609 complex also provides a structural basis for designing novel inhibitors to specifically block CaMKKβ and related protein kinases.[2] |
| 分子式 |
C21H14N2O5
|
|
|---|---|---|
| 分子量 |
374.35
|
|
| 精确质量 |
374.09
|
|
| 元素分析 |
C, 67.38; H, 3.77; N, 7.48; O, 21.37
|
|
| CAS号 |
1173022-21-3
|
|
| 相关CAS号 |
STO-609;52029-86-4
|
|
| PubChem CID |
16760660
|
|
| 外观&性状 |
Typically exists as solid at room temperature
|
|
| LogP |
3.381
|
|
| tPSA |
108.97
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
1
|
|
| 重原子数目 |
28
|
|
| 分子复杂度/Complexity |
596
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
C1=CC=C2C(=C1)N=C3C4=CC=C(C5=C4C(=CC=C5)C(=O)N23)C(=O)O.CC(=O)O
|
|
| InChi Key |
WNRSTFUVBWNELX-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C19H10N2O3.C2H4O2/c22-18-13-5-3-4-10-11(19(23)24)8-9-12(16(10)13)17-20-14-6-1-2-7-15(14)21(17)18;1-2(3)4/h1-9H,(H,23,24);1H3,(H,3,4)
|
|
| 化学名 |
acetic acid;11-oxo-3,10-diazapentacyclo[10.7.1.02,10.04,9.016,20]icosa-1(20),2,4,6,8,12,14,16,18-nonaene-17-carboxylic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6713 mL | 13.3565 mL | 26.7130 mL | |
| 5 mM | 0.5343 mL | 2.6713 mL | 5.3426 mL | |
| 10 mM | 0.2671 mL | 1.3356 mL | 2.6713 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。