| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Four beagle dogs/sex/group were dosed by gavage at 0, 1, 3, or 6 mg/kg/day for 1 year with Sulfoxaflor, purity 96.6%... Periodic blood and urine collection was done to estimate tissue clearance and excretion patterns of parent sulfoxaflor. NOEL = 3 mg/kg/day, based on food consumption decrements in the first 2-3 weeks on study in both sexes, on increased soft or watery feces in males, and on a modest increase in tan vomitus in both sexes. Plasma concentration of parent sulfoxaflor over time peaked at about 2 hours, with about 40% of peak residues at 24 hours, with no obvious effect of sex on plasma levels. An estimated terminal plasma half-life of sulfoxaflor was about 20 hours for either sex. Typically about 60% to 80% of administered dose was recovered as parent sulfoxaflor in 24-hr urine collections. When rats were orally dosed with labelled sulfoxaflor, approximately 93% of the dose was eliminated in the urine and faeces as parent sulfoxaflor. The main metabolite in urine was a glucuronide conjugate of sulfoxaflor metabolite X11721061, accounting for approximately 2-4% of the dose. Several other unidentified minor components, each less than 1% of the administered dose, were present in the urine and fecal samples... /MILK/ When lactating goats were orally dosed with labelled sulfoxaflor at 12.2 ppm in the diet, approximately 4% of the dose appeared in the milk and 3% in the tissues. Rats were administered sulfoxaflor, purity 95.6%... containing suitable specific activity of C14 ring-labeled sulfoxaflor, purity 97.6%, with over 2% XR-208 ketone byproduct. Groups were (1) a single low (5 mg/kg) gavage dose, (2) a single high (100 mg/kg) gavage dose, (3) daily low doses of 5 mg/kg unlabeled sulfoxaflor, followed on day 15 with 5 mg/kg labeled sulfoxaflor, or (4) a single iv dose of 5 mg/kg. Sacrifice was at 168 hr. Generally label was measured in blood, excreta, and tissues to assess kinetics. Metabolic residues were determined in urine and feces. Tissue residues were very low or below detection levels. About 65-70% of administered dose was excreted in urine within 12 hr of dosing, with continuing rapid clearing. Feces comprised 4-5% of administered dose within 24 hr. Carbon trap results were normally below detection. There was no apparent effect of 14-day pre-treatment with low doses. In all of these cases, about 92% of administered dose was excreted in urine and 5-7% in feces, with no apparent sex difference and no difference due to dose level. Intravenous dosing yielded 97-101% of estimated administered dose in urine, and 6-9% in feces. As with the gavage treatments, residues in blood or internal organs were minimal after 7 days. Tmax estimates in plasma for single gavage treatments were typically 1-2 hr. Plasma elimination first phase t1/2 regardless of dose or route ranged from 4-6 hr, with second elimination phases of about 40 hr duration. Patterns were comparable based on RBC's except that second phase t1/2 ranged from about 50 to 75 hours. Two large adjacent peaks, identified as the two diastereomers of parent sulfoxaflor, comprised the bulk of radioactivity in excreta. The first of these peaks eluting in urine ("Peak F") comprised about 53% of administered dose, compared to about 37% for the second peak ("Peak G"). This ratio often exceeded 2:1 for the smaller amounts of radioactivity in feces. These results suggest that the labeled test article was not a racemic mixture, and further that metabolism of one isomer might be favored over the other. Other than one glucuronide (designated X11721061), no other metabolite exceeded 1% of administered dose. The glucuronide evidently involved cleavage between the sulfur and the methylene carbon of sulfoxaflor prior to conjugation at the cleavage site. None of the lesser peaks was characterized. This conjugate was only quantifiable in urine... For more Absorption, Distribution and Excretion (Complete) data for Sulfoxaflor (7 total), please visit the HSDB record page. Metabolism / Metabolites Residue levels in milk reached a plateau during days 3-4 of the dosing phase, at about 0.2 mg/kg eq. Similar (14)C levels were found in liver, kidney, milk, and muscle; while lower levels were reported in fat tissues. This study demonstrated that X11719474 is not metabolized in goats: no metabolites were identified, and the radioactivity found in all tissues was from X11719474. When laying hens were orally dosed with labelled sulfoxaflor metabolite X11719474 at 11.8 ppm in the feed, approximately 0.5% of the applied dose was recovered in the combined eggs, fat, and tissues. Similar (14)C levels were noted in liver, muscle, and egg; lower levels were found in fat tissues. Approximately 92% of the dose was recovered from the excreta, and 0.3% in the cage rinse. Residue levels in eggs reached a plateau by day 4 of the dosing phase, with no compounds other than X11719474 being identified. This study demonstrated that X11719474 is not metabolized in hens: no metabolites were identified and the radioactivity found in all tissues was from X11719474. /Sulfoxaflor metabolite/ All metabolites /tested/ were less toxic than the parent compound, except for X11519540, which had higher acute and higher short-term toxicity than the parent. X11721061, a plant and animal (rat) metabolite of sulfoxaflor, was of low acute oral toxicity in rats (LD50 > 2000 mg/kg bw) and showed no genotoxic potential in vitro in mammalian or microbial test systems. In a 28-day oral toxicity study in rats, the NOAEL was 3000 ppm (equal to 236 mg/kg bw per day), based on reduced feed consumption at 8000 ppm (equal to 622 mg/kg bw per day). /Sulfoxaflor metabolite/ Biological Half-Life Four beagle dogs/sex/group were dosed by gavage at 0, 1, 3, or 6 mg/kg/day for 1 year with Sulfoxaflor... An estimated terminal plasma half-life of sulfoxaflor was about 20 hours for either sex. .../In/ F344/DuCrl rats...plasma samples were evaluated to find an elimination t1/2 of 8-9 hours. Rats were administered sulfoxaflor, purity 95.6%... containing suitable specific activity of C14 ring-labeled sulfoxaflor, purity 97.6%, with over 2% XR-208 ketone byproduct. Groups were (1) a single low (5 mg/kg) gavage dose, (2) a single high (100 mg/kg) gavage dose, (3) daily low doses of 5 mg/kg unlabeled sulfoxaflor, followed on day 15 with 5 mg/kg labeled sulfoxaflor, or (4) a single iv dose of 5 mg/kg. ... Plasma elimination first phase t1/2 regardless of dose or route ranged from 4-6 hr, with second elimination phases of about 40 hr duration. Patterns were comparable based on RBC's except that second phase t1/2 ranged from about 50 to 75 hours. ... |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Sulfoxaflor is a white powder with a sharp odor that is registered for pesticide use in the USA but approved pesticide uses may change periodically and so federal, state and local authorities must be consulted for currently approved uses. Sulfoxaflor is the first member of a new class of insecticides, the sulfoximines, and is a highly efficacious activator of the nicotinic acetylcholine receptor (nAChR) in insects. HUMAN EXPOSURE AND TOXICITY: Sulfoxaflor was shown to have no agonism on human fetal or adult muscle nAChRs. The data support the conclusion that the developmental effects of sulfoxaflor in rats are mediated via sustained agonism on the fetal muscle nAChR during late fetal development and are considered not relevant to humans. ANIMAL STUDIES: Toxicity and mechanistic studies in rats, rabbits, dogs and mice indicate that sulfoxaflor is an activator of the mammalian nAChR as well, but to a much lesser degree and in a species-specific manner. The nervous system and liver are the target organ systems of sulfoxaflor and its major metabolites resulted in hepatotoxicity; including liver weight and enzyme changes, hypertrophy, proliferation, and hepatocellular adenocarcinomas in subchronic and chronic studies in rodents. Developmental toxicity, manifested as skeletal abnormalities and neonatal deaths, was observed in rats only. The skeletal abnormalities, including forelimb flexure, bent clavicles, and hindlimb rotation, likely resulted from skeletal muscle contraction due to activation of the skeletal muscle nAChR in utero. Contraction of the diaphragm, also related to skeletal muscle nAChR activation, prevented normal breathing in neonates and resulted in increased mortality in the reproduction studies. Oral studies of mid- and high-dose sulfoxaflor resulted in decreased food consumption and subsequent decreased body weight as well as changes in the male reproductive system. Effects in the male reproductive organs were observed in the carcinogenicity study in rats that included increased testicular and epididymal weights, atrophy of seminiferous tubules, and decreased secretory material in the coagulating glands, prostate, and seminal vesicles. Additionally, there was an increased incidence of interstitial cell (Leydig cell) tumors, considered secondary to loss of normal testicular function. At the highest dose tested, muscle tremors and twitches, convulsions, hindlimb splaying, increased lacrimation and salivation, decreased pupil size and response to touch, gait abnormalities and decreased rectal temperature were observed. Decreased motor activity was also observed in the mid- and high-dose groups. Sulfoxaflor was negative for chromosomal aberrations with and without metabolic activation, and tested negative in Salmonella typhimurium strains TA 1535, TA 100, TA 1537, TA 98, and E. coli: WP2 uvrA in a standard reverse mutation assay. Interactions Investigators made isolated phrenic nerve-hemidiaphragm preparations from newborn rats. System included a muscle strain gauge transducer, a stimulating electrode fixed to the phrenic nerve, and tissue preparation anchored in a vessel which allowed changing of test solutions on demand. It was postulated that sulfoxaflor would be an agonist toward the embryonic nicotinic acetylcholine receptor (nAChR). Initial tests confirmed muscle contraction and reduced twitch response to phrenic nerve stimulation at 100 uM ACh, with return to normal upon solution wash. Ten uM tubocurarine rapidly reduced muscle tension and twitch response. Sulfoxaflor caused muscle contraction similar to ACh: at 100 uM sulfoxaflor there was no evident change on nerve-stimulated muscle twitch, whereas at 1 mM sulfoxaflor the twitch response was reduced to about 34% of normal. Ten uM tubocurarine administered simultaneously with 1 mM sulfoxaflor blocked muscle contraction by about 50%. Pre-incubation with 10 uM tubocurarine essentially eliminated the muscle contraction by 1 mM sulfoxaflor. Prolonged exposure to 1 mM sulfoxaflor (7 min) led to sustained muscle contraction and reduction in twitch response, which was reversible on washing. Tests support the hypothesis that neonatal death could have arisen from diaphragm failure by impairing respiration. Non-Human Toxicity Values LD50 Rat oral 1000 mg/kg bw LD50 Rat dermal >5000 mg/kg bw LC50 Rat inhalation >2.09 mg/L (4 hr, nose-only exposure) |
| 参考文献 | |
| 其他信息 |
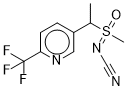
[methyl(oxido){1-[6-(trifluoromethyl)pyridin-3-yl]ethyl}-lambda(6)-sulfanylidene]cyanamide is a member of the class of pyridines that is 5-ethyl-2-trifluoromethylpyridine in which the ethyl group is substituted at position 1 by an N-cyano-S-methylsulfonimidoyl group. The insecticide sulfoxalor is a mixture of the four possible stereoisomers arising from the two tetrahedral stereocentres. It is a member of pyridines, a sulfoximide, a nitrile and an organofluorine compound.
Mechanism of Action This report effectively applies a mode of action sequence... to explain the non-significantly elevated incidence of preputial gland carcinomas in the rat combined study. The sequence proposed is: (1) increased dopamine release from the hypothalamus, causing (2) reduced prolactin secretion from the pituitary, in turn causing (3) reduced stimulation of prolactin receptors on Leydig cells, which reduces LH receptor density in Leydig cells, causing (4) downregulation of LH receptor gene expression, causing (5) transient decrease in serum testosterone, leading to (6) increased serum LH levels, causing (7) increased serum testosterone via hypothalamic/pituitary/gonadal feedback loop, leading to (8) increased preputial gland carcinomas due to slightly increased higher circulating testosterone. Authors note that item (3) does not appear to relate to humans (which statement is consistent with published literature)... Sulfoxaflor (X11422208), a novel agricultural molecule, induced fetal effects (forelimb flexure, hindlimb rotation, and bent clavicle) and neonatal death in rats at high doses (= 400 ppm in diet); however, no such effects occurred in rabbit dietary studies despite achieving similar maternal and fetal plasma exposure levels. Mode-of-action (MoA) studies were conducted to test the hypothesis that the effects in rats had a single MoA induced by sulfoxaflor agonism on the fetal rat muscle nicotinic acetylcholine receptor (nAChR). The studies included cross-fostering and critical windows of exposure studies in rats, fetal... and adult... rat and human muscle nAChR in vitro agonism experiments, and neonatal rat phrenic nerve-hemidiaphragm contracture studies. The weight of evidence from these studies supported a novel MoA where sulfoxaflor is an agonist to the fetal, but not adult, rat muscle nAChR and that prolonged agonism on this receptor in fetal/neonatal rats causes sustained striated muscle contracture resulting in concomitant reduction in muscle responsiveness to physiological nerve stimulation. Fetal effects were inducible with as little as 1 day of exposure at the end of gestation, but were rapidly reversible after birth, consistent with a pharmacological MoA. With respect to human relevance, sulfoxaflor was shown to have no agonism on human fetal or adult muscle nAChRs. Taken together, the data support the hypothesis that the developmental effects of sulfoxaflor in rats are mediated via sustained agonism on the fetal muscle nAChR during late fetal development and are considered not relevant to humans. |
| 分子式 |
C10H10F3N3OS
|
|---|---|
| 分子量 |
277.2652
|
| 精确质量 |
277.05
|
| CAS号 |
946578-00-3
|
| PubChem CID |
16723172
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.34g/cm3
|
| 沸点 |
363.8ºC at 760 mmHg
|
| 熔点 |
112 °C (99.7% purity)
|
| 闪点 |
173.8ºC
|
| 折射率 |
1.519
|
| LogP |
3.605
|
| tPSA |
74.49
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
18
|
| 分子复杂度/Complexity |
459
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
ZVQOOHYFBIDMTQ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C10H10F3N3OS/c1-7(18(2,17)16-6-14)8-3-4-9(15-5-8)10(11,12)13/h3-5,7H,1-2H3
|
| 化学名 |
N-(methyloxido(1-(6-(trifluoromethyl)-3-pyridinyl)ethyl)-lambda4-sulfanylidene)cyanamide
|
| 别名 |
Sulfoxaflor GF 2372 XDE-208GF-2032XDE 208GF 2032
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
|---|
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.6066 mL | 18.0330 mL | 36.0659 mL | |
| 5 mM | 0.7213 mL | 3.6066 mL | 7.2132 mL | |
| 10 mM | 0.3607 mL | 1.8033 mL | 3.6066 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。