| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 靶点 |
vasopressin V1 receptor
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following a 1 mg IV injection of terlipressin acetate in patients with HRS-1, the median Cmax, AUC24h and Cave of terlipressin at steady-state were 70.5 ng/mL, 123 ng × hr/mL and 14.2 ng/mL, respectively. The median Cmax, AUC24h and Cave of lypressin were 1.2 ng/mL, 11.2 ng × hr/mL and 0.5 ng/mL, respectively. Terlipressin and lypressin exhibit linear pharmacokinetics in healthy subjects. Plasma concentrations of terlipressin demonstrate proportional increases with the dose administered. Less than 1% of terlipressin and <0.1% of lysine-vasopressin is excreted in urine in healthy subjects. The volume of distribution of terlipressin was 6.3 L and 1370 L for lysine-vasopressin. The clearance of terlipressin was 27.4 L/hr and 318 L/hr for lysine-vasopressin. Clearance of terlipressin in HRS-1 patients increased with body weight, while body weight had no effect on the clearance of lysine-vasopressin. Metabolism / Metabolites The N-terminal glycyl residues of terlipressin is cleaved by various tissue peptidases to release its pharmacologically active metabolite, [lypressin] or lysine-vasopressin. Once formed, lypressin is undergoes various peptidase-mediated metabolic pathways in body tissues. Terlipressin is not metabolized in the blood or plasma. Due to the ubiquitous nature of peptidases in body tissues, it is unlikely that the metabolism of terlipressin will be affected by disease state or other drugs. Biological Half-Life The terminal half-life of terlipressin was 0.9 hours and 3.0 hours for lysine-vasopressin. |
| 参考文献 |
|
| 其他信息 |
Pharmacodynamics
Terlipressin mimics the biological effects of endogenous vasopressin, but it displays increased selectivity for the V1 receptor and a longer half-life than vasopressin. These pharmacokinetic and molecular properties of terlipressin give it several advantages, such as the prevention of rebound hypotension when the drug is stopped and convenience in patients with limited intravenous access. Terlipressin increases arterial pressure (diastolic, systolic, and mean) and decreases heart rate in patients with hepatorenal syndrome type 1 (HRS-1). After the administration of a single 0.85 mg dose of terlipressin in patients with HRS-1, cardiovascular effects were observed within five minutes after dosing and were maintained for at least six hours after dosing. The maximum change in blood pressure and heart rate occurred at 1.2 to two hours post-dose. |
| 分子式 |
C54H78N16O17S2
|
|---|---|
| 分子量 |
1287.433
|
| 精确质量 |
1346.538
|
| 元素分析 |
C, 50.38; H, 6.11; N, 17.41; O, 21.13; S, 4.98
|
| CAS号 |
914453-96-6
|
| 相关CAS号 |
Terlipressin diacetate; 1884420-36-3; Terlipressin; 14636-12-5
|
| PubChem CID |
72081
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
-5.8
|
| tPSA |
563
|
| 氢键供体(HBD)数目 |
16
|
| 氢键受体(HBA)数目 |
19
|
| 可旋转键数目(RBC) |
25
|
| 重原子数目 |
85
|
| 分子复杂度/Complexity |
2380
|
| 定义原子立体中心数目 |
8
|
| SMILES |
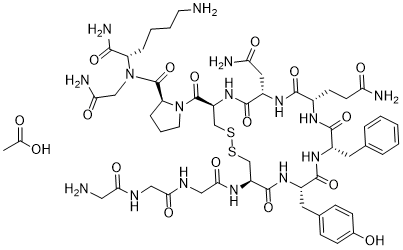
S1C[C@@H](C(N2CCC[C@H]2C(N[C@H](C(NCC(N)=O)=O)CCCCN)=O)=O)NC([C@H](CC(N)=O)NC([C@H](CCC(N)=O)NC([C@H](CC2C=CC=CC=2)NC([C@H](CC2C=CC(=CC=2)O)NC([C@H](CS1)NC(CNC(CNC(CN)=O)=O)=O)=O)=O)=O)=O)=O.OC(C)=O
|
| InChi Key |
BYDVFOPTAIPAGA-LCGYVTRFSA-N
|
| InChi Code |
InChI=1S/C52H74N16O15S2.C2H4O2/c53-17-5-4-9-31(45(76)60-23-41(57)72)63-51(82)38-10-6-18-68(38)52(83)37-27-85-84-26-36(61-44(75)25-59-43(74)24-58-42(73)22-54)50(81)65-34(20-29-11-13-30(69)14-12-29)48(79)64-33(19-28-7-2-1-3-8-28)47(78)62-32(15-16-39(55)70)46(77)66-35(21-40(56)71)49(80)67-37;1-2(3)4/h1-3,7-8,11-14,31-38,69H,4-6,9-10,15-27,53-54H2,(H2,55,70)(H2,56,71)(H2,57,72)(H,58,73)(H,59,74)(H,60,76)(H,61,75)(H,62,78)(H,63,82)(H,64,79)(H,65,81)(H,66,77)(H,67,80);1H3,(H,3,4)/t31-,32-,33-,34-,35-,36-,37-,38-;/m0./s1
|
| 化学名 |
acetic acid;(2S)-1-[(4R,7S,10S,13S,16S,19R)-19-[[2-[[2-[(2-aminoacetyl)amino]acetyl]amino]acetyl]amino]-7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-13-benzyl-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]-N-[(2S)-6-amino-1-[(2-amino-2-oxoethyl)amino]-1-oxohexan-2-yl]pyrrolidine-2-carboxamide
|
| 别名 |
Gly-Gly-Gly-8-Lys-vasopressin; TGLVP; triglycyl lysine vasopressin; Terlipressin; Terlipressin acetate; trade names: Glypressin; Teripress
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.7767 mL | 3.8837 mL | 7.7674 mL | |
| 5 mM | 0.1553 mL | 0.7767 mL | 1.5535 mL | |
| 10 mM | 0.0777 mL | 0.3884 mL | 0.7767 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Treatment of hepatorenal syndrome with terlipressin infusion adjusted to hemodynamic response
CTID: null
Phase: Phase 4 Status: Completed
Date: 2012-02-08
|
|
|
|
|