| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 体外研究 (In Vitro) |
甲硫哒嗪(0.01-100 μM;48 小时)以浓度依赖性方式降低 NCI-N87 和 AGS 细胞活力 [2]。硫利达嗪(15 μM;24 小时)会降低子宫内膜癌细胞(HEC-1-A 和 KLE)和宫颈癌细胞(HeLa、Caski 和 C33A)的细胞活力 [4]。硫利达嗪(1-15 μM;24-48 h)通过线粒体途径和线粒体凋亡途径导致胃癌细胞死亡[2]。在宫颈癌和子宫内膜癌细胞中,硫利达嗪(15 μM;24 小时)可促进 G1 细胞周期停滞并干扰 PI3K/Akt 通路以调节细胞周期进展 [4]。当使用硫利达嗪时,不能产生具有多重耐药性且对抗生素敏感的鲍曼不动杆菌菌株[3]。
|
|---|---|
| 体内研究 (In Vivo) |
当给予硫利达嗪(25 mg/kg;腹腔内每 3 天一次,持续 3 周)时,荷瘤小鼠的存活时间延长,并且肿瘤内多能胚胎癌细胞 (EC) 的数量减少 [5]。皮下注射硫利达嗪 (1.0-5.0 mg/kg) 可降低口腔习惯并选择性抑制重复头部摆动 [1]。
|
| 细胞实验 |
细胞活力测定[1]
细胞类型: NCI-N87 和 AGS 细胞。 测试浓度:0.01、0.1、0.5、1、5、10、20、50、100 μM。 孵化持续时间:48小时。 实验结果:证明对胃癌细胞具有细胞毒性。 蛋白质印迹分析[1] 细胞类型: NCI-N87 和 AGS 细胞 测试浓度: 1、5、10、 15μM。 孵化持续时间:24、48 小时。 实验结果: caspase-9、caspase-8 和 caspase-3 前体蛋白下调。 |
| 动物实验 |
Animal/Disease Models: Nude mice and Rag2KO mice were injected with iPS cells or NT2D1 cells [5].
Doses: 25 mg/kg. Route of Administration: IP every 3 days for 3 weeks. Experimental Results: The number of OCT4-expressing cells in malignant teratocarcinoma was diminished and the survival period of tumor-bearing mice was prolonged. Has no effect on fertility. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
60% Experimental studies in animals and in vitro have demonstrated that thioridazine has affinity for melanin granules and tends to accumulate in close association with uveal pigment ... . ... Pharmacokinetics and metabolism ... similar ... to chlorpromazine, but strong anticholinergic action of thioridazine on the gut may modify its own absorption ... . Concentrations of thioridazine in plasma are relatively high (hundreds of nanograms per milliliter), possibly owing to its relative hydrophilicity ... . In 48 patients taking thiordazine the mean amount not bound to serum proteins was 0.15%, that of the side-chain sulfoxide 1.66%, side-chain sulfone 1.17%, and ring sulfoxide 1.7%. Thioridazine and metabolites were measured in brain, liver, and kidney specimens, obtained postmortem from two subjects whose deaths were related to acute intoxication with thioridazine, by gas-liquid chromatography. Although the absolute concentration measured for thioridazine and metabolites differed in the two cases, the metabolic pattern for each tissue, expressed in terms of the percentage of total drug in each tissue, was quite similar. The brain, liver, and kidney metabolic patterns, however, are in sharp contrast to the plasma metabolite patterns observed for subjects on a therapeutic regimen of thioridazine. As this example demonstrates, postmortem specimens are a valuable (but seldom used) source of human pharmacological data. For more Absorption, Distribution and Excretion (Complete) data for Thioridazine (10 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic Major metabolites include sulfoxy products at ring position 5 (inactive) or at substituent at position 2 (including active metabolite mesoridazine). Demethylation of piperidine ring is very rapid ... . Although the exact metabolic fate of phenothiazines has not been clearly established, the drugs are extensively metabolized, principally in the liver via hydroxylation, oxidation, demethylation, sulfoxide formation, and conjugation with glucuronic acid; metabolic alterations in the side chain also may occur. /Phenothiazine General Statement/ Most metabolites of phenothiazines are pharmacologically inactive; however, certain metabolites (eg, 7-hydroxychlorpromazine, mesoridazine) show moderate pharmacologic activity and may contribute to the action of the drugs. There is limited evidence to indicate that some phenothiazines (eg, chlorpromazine) may induce their own metabolism. /Phenothiazine General Statement/ Thioridazine and metabolites were determined by a selective HPLC technique in blood from five post-mortem cases; two deaths attributed to drug overdose and three deaths due to natural causes or trauma. Additionally, total thioridazine-like compounds were determined in these blood samples and liver specimens by a nonspecific fluorometric technique. Blood concentrations were: thioridazine, 0.78-8.85 mg/L; mesoridazine, 0.52-26.8 mg/L; and sulforidazine, 0.00-0.87 mg/L. Thioridazine-5-sulfoxide stereoisomeric DL,LD, and DD,LL pair concentrations ranged from 0.02-0.56 and 0.03-0.83 mg/L, respectively. Thioridazine metabolite profiles were not helpful in differentiating therapeutic administration from severe overdose. Liver appears to be the specimen of choice in the assessment of thioridazine overdose. For more Metabolism/Metabolites (Complete) data for Thioridazine (8 total), please visit the HSDB record page. Thioridazine has known human metabolites that include N-desmethylthioridazine, Thioridazine 2-sulfoxide, and Thioridazine 5-sulfoxide. Sulphoridazine is a known human metabolite of schembl149458. Hepatic Half Life: 21-25 hours Biological Half-Life 21-25 hours Serum half-life of thioridazine has been estimated to range from about 6 to over 40 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Thioridazine blocks postsynaptic mesolimbic dopaminergic D1 and D2 receptors in the brain; blocks alpha-adrenergic effect, depresses the release of hypothalamic and hypophyseal hormones and is believed to depress the reticular activating system thus affecting basal metabolism, body temperature, wakefulness, vasomotor tone, and emesis. Hepatotoxicity Liver test abnormalities have been reported to occur in a high proportion of patients on long term phenothiazine therapy, but elevations are uncommonly above 3 times the upper limit of normal. The aminotransferase abnormalities are usually mild, asymptomatic and transient, reversing even with continuation of medication. Rare instances of clinically apparent acute liver injury have been reported due to thioridazine, with some resemblance to cases of chlorpromazine jaundice. The onset of jaundice occurred within a few weeks to several months of therapy and the pattern of serum enzyme elevations was typically cholestatic, although hepatocellular patterns have also been reported. Immunoallergic manifestations (fever, rash and eosinophilia) were not prominent and autoantibodies were not detected. Some cases were associated with agranulocytosis which is a rare but known complication of the phenothiazines. Likelihood score: B (likely but rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because there is no published experience with thioridazine during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Phenothiazines cause galactorrhea in 26 to 40% of female patients. Hyperprolactinemia appears to be the cause of the galactorrhea. There is some evidence that thioridazine increases serum prolactin to a greater extent than other phenothiazines. The hyperprolactinemia is caused by the drug's dopamine-blocking action in the tuberoinfundibular pathway. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding 95% Toxicity Data LD50=956-1034 mg/kg (Orally in rats). Interactions QT interval-prolonging medications, including cisapride, erythromycin, and quinidine /may produce/ additive QT interval prolongation increasing the risk of developing cardiac arrhythmias when /concurrently administered with phenothiazines/. /Phenothiazines/ Concurrent use /of other photosensitizing medications/ with phenothiazines may cause additive photosensitizing effects. In addition, concurrent use of systemic methoxsalen, trixsalen, or tetracyclines with phenothiazines may potentiate intraocular photochemical damage to the choroid, retina, or lens. /Phenothiazines/ Prior administration of phenothiazines may decrease the pressor effect and shorten the duration of action of phenylephrine. /Phenothiazines/ In addition to increased CNS and respiratory depression, concurrent use /of opiod (narcotic) analgesics/ with phenothiazines increases orthostatic hypotension and increases the risk of severe constipation, which may lead to paralytic ileus, and/or urinary retention. /Phenothiazines/ For more Interactions (Complete) data for Thioridazine (30 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral 995 mg/kg |
| 参考文献 |
[1]. Tschanz JT, et, al. Atypical antipsychotic drugs block selective components of amphetamine-induced stereotypy. Pharmacol Biochem Behav. 1988 Nov;31(3):519-22.
[2]. Mu J, et, al. Thioridazine, an antipsychotic drug, elicits potent antitumor effects in gastric cancer. Oncol Rep. 2014 May;31(5):2107-14. [3]. Kang S, et, al. Thioridazine induces apoptosis by targeting the PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells. Apoptosis. 2012 Sep;17(9):989-97. [4]. Loehr AR, et, al. Targeting Cancer Stem Cells with Differentiation Agents as an Alternative to Genotoxic Chemotherapy for the Treatment of Malignant Testicular Germ Cell Tumors. Cancers (Basel). 2021 Apr 23;13(9):2045. |
| 其他信息 |
Therapeutic Uses
Antipsychotic Agents, Phenothiazine; Dopamine Antagonists Thioridazine is indicated for the management of schizophrenic patients who fail to respond adequately to treatment with other antipsychotic drugs. Due to the risk of significant, potentially life threatening, proarrhythmic effects with thioridazine treatment, thioridazine should be used only in patients who have failed to respond adequately to treatment with appropriate courses of other antipsychotic drugs, either because of insufficient effectiveness or the inability to achieve an effective dose due to intolerable adverse effects from those drugs. Consequently, before initiating treatment with thioridazine, it is strongly recommended that a patient be given at least two trials, each with a different antipsychotic drug product, at an adequate dose, and for an adequate duration. /Included in US product label/ The prescriber should be aware that thioridazine has not been systematically evaluated in controlled trials in treatment refractory schizophrenic patients and its efficacy in such patients is unknown. /Included in US product label/ The US Food and Drug Administration (FDA) currently advises clinicians that antipsychotic agents are not approved for the treatment of dementia-related psychosis. FDA further advises clinicians that no drugs currently are approved for the treatment of patients with dementia-associated psychosis and that other management options should be considered in such patients. /Phenothiazine General Statement/ Drug Warnings ... Extrapyramidal reactions ... fairly common, usually 3 types ... Parkinsonian-like syndrome ... dystonia and dyskinesia, including torticollis, tics, and other involuntary muscle movements ... akathisia, shown by restlessness ... hyperreflexia, reported in newborn ... ./Phenothiazines/ Thioridazine has been shown to prolong the QTc interval in a dose related manner, and drugs with this potential, including thioridazine, have been associated with Torsades de pointes type arrhythmias and sudden death. Due to its potential for significant, possibly life threatening, proarrhythmic effects, thioridazine should be reserved for use in the treatment of schizophrenic patients who fail to show an acceptable response to adequate courses of treatment with other antipsychotic drugs, either because of insufficient effectiveness or the inability to achieve an effective dose due to intolerable adverse effects from those drugs Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (eg, heart failure, sudden death) or infectious (eg, pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Thioridazine hydrochloride is not approved for the treatment of patients with dementia-related psychosis In common with other phenothiazines, thioridazine is contraindicated in severe central nervous system depression or comatose states from any cause including drug induced central nervous system depression. It should also be noted that hypertensive or hypotensive heart disease of extreme degree is a contraindication of phenothiazine administration. For more Drug Warnings (Complete) data for Thioridazine (48 total), please visit the HSDB record page. Pharmacodynamics Thioridazine is a trifluoro-methyl phenothiazine derivative intended for the management of schizophrenia and other psychotic disorders. Thioridazine has not been shown effective in the management of behaviorial complications in patients with mental retardation. |
| 分子式 |
C21H26N2S2
|
|---|---|
| 分子量 |
370.573
|
| 精确质量 |
370.153
|
| CAS号 |
50-52-2
|
| 相关CAS号 |
Thioridazine hydrochloride;130-61-0;Thioridazine-d3 hydrochloride;1189928-36-6
|
| PubChem CID |
5452
|
| 外观&性状 |
Crystals from acetone
Colorless crystals |
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
515.7±50.0 °C at 760 mmHg
|
| 熔点 |
72-74°
|
| 闪点 |
265.7±30.1 °C
|
| 蒸汽压 |
0.0±1.3 mmHg at 25°C
|
| 折射率 |
1.677
|
| LogP |
6.13
|
| tPSA |
57.08
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
25
|
| 分子复杂度/Complexity |
432
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
KLBQZWRITKRQQV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C21H26N2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3
|
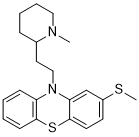
| 化学名 |
10-[2-(1-methylpiperidin-2-yl)ethyl]-2-methylsulfanylphenothiazine
|
| 别名 |
Thioridazine Melleril Mellaril
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6985 mL | 13.4927 mL | 26.9855 mL | |
| 5 mM | 0.5397 mL | 2.6985 mL | 5.3971 mL | |
| 10 mM | 0.2699 mL | 1.3493 mL | 2.6985 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。