| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 1g |
|
||
| Other Sizes |
| 靶点 |
GIP (glucose-dependent insulin nutritive polypeptide); GLP-1 (glucagon-like peptide-1) receptor
Tirzepatide is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist. - EC₅₀ at human GIPR: 0.068 nM - EC₅₀ at human GLP-1R: 0.12 nM - Bias toward GIPR activation (GIPR potency ≈ 5× GLP-1R) [2][3] |
|---|---|
| 体外研究 (In Vitro) |
在减肥和血糖控制方面,替西帕肽(LY3298176)的疗效明显高于杜拉鲁肽[1]。替西帕肽是GIPR和GLP-1R的不平衡激动剂,并且在GLP-1R处显示有偏向的信号传导。替西帕肽与GLP-1R不同地诱导GIPR的内化。[2].
Tirzepatide (LY3298176)是一种双重GIP和GLP-1受体激动剂,正在开发用于治疗2型糖尿病(T2DM)、肥胖和非酒精性脂肪性肝炎。T2DM的早期试验表明,替西肽比选择性GLP-1受体激动剂更能改善临床结果。因此,我们假设替西帕肽的综合效力和信号特性为改善广泛的代谢控制提供了独特的药理学特征。在这里,我们建立的方法来计算每个受体占用临床有效剂量的药物。这一分析揭示了替西肽对GIP受体比GLP-1受体更大程度的参与,证实了不平衡的作用机制。药理学上,信号研究表明,Tirzepatide模仿天然GIP对GIP受体的作用,但在GLP-1受体上表现出偏向于cAMP的产生而不是β-抑制蛋白的募集,与GLP-1相比,其驱动GLP-1受体内化的能力较弱。原代胰岛实验显示β-阻滞1限制胰岛素对GLP-1的反应,但不限制GIP或替西帕肽,提示替西帕肽的偏激激动作用增强胰岛素分泌。对GIP受体的不平衡,加上GLP-1受体的不同信号特性,可能共同解释了该研究药物有希望的疗效。[2] Tirzepatide 是由GIP类似物通过C20脂肪二酸连接子与GLP-1类似物缀合而成的39肽,支持每周一次给药。 [3] 作用机制:通过双受体激活协同增强胰岛素分泌、抑制胰高血糖素、促进饱腹感。 [1][2] 在GLP-1R显示偏向信号传导(优先激活cAMP而非β-抑制蛋白募集)。 [2] |
| 体内研究 (In Vivo) |
Tirzepatide (LY3298176) 在血糖控制和体重减轻方面显示出比度拉鲁肽更好的功效[1]。通过对小鼠的长期给药,LY3298176有效地降低了体重和食物摄入量;这些作用明显大于GLP-1受体激动剂的作用。[3]
替西帕肽显著改善糖尿病大鼠受损的糖耐量、空腹血糖水平和胰岛素水平。然后,替西帕肽显著减轻了糖尿病海马的空间学习和记忆障碍,抑制了Aβ的积累,防止了结构损伤,促进了突触蛋白的合成,并增加了树突棘的形成。此外,在糖尿病大鼠接受替西帕肽治疗后,与炎症信号通路有关的信号分子的一些异常变化被正常化。最后,替西帕肽恢复了PI3K/Akt/GSK3β信号通路。[4] 背景:糖尿病患者的典型症状之一是记忆障碍,随后是逐渐的认知能力下降,目前尚无有效的治疗方法。抗糖尿病肠促胰岛素激素葡萄糖依赖性胰岛素性多肽(GIP)和胰高血糖素样肽-1 (GLP-1)在AD动物模型中被证明具有高度的神经保护作用。我们想找出GLP-1/GIP双激动剂Tirzepatide是如何影响糖尿病的空间学习记忆障碍的。方法:采用高脂饮食和链脲佐菌素诱导的糖尿病大鼠腹腔注射Tirzepatide/替西帕肽(1.35 mg/kg),每周1次。采用Morris水迷宫试验、免疫荧光和Western blot分析评估其保护作用。定量树突棘采用高尔基染色法。结果:替西帕肽显著改善糖尿病大鼠糖耐量、空腹血糖和胰岛素水平。替西帕肽显著减轻糖尿病海马空间学习记忆障碍,抑制Aβ积累,防止结构损伤,促进突触蛋白合成,增加树突棘形成。此外,替西肽治疗后,糖尿病大鼠炎症信号通路相关信号分子的一些异常变化趋于正常化。最后,Tirzepatide恢复了PI3K/Akt/GSK3β信号通路。结论:替西帕肽对空间学习记忆障碍具有明显的保护作用,其机制可能与调节胰岛素抵抗异常和炎症反应有关。[4] 在糖尿病(db/db)小鼠中,每周皮下注射Tirzepatide(1-10 nmol/kg)4周,较溶剂组降低HbA1c 1.5-2.5%、减轻体重10-25%(p<0.01),疗效优于同等剂度的度拉糖肽(GLP-1激动剂)。 [3] 2期临床试验(T2D患者):Tirzepatide(1-15 mg/周,皮下注射)26周后,HbA1c降低1.6-2.4%,体重减轻2.0-11.3 kg(剂量依赖性),疗效优于度拉糖肽1.5 mg(HbA1c -1.1%;体重-2.7 kg)。 [1] 在糖尿病大鼠中,Tirzepatide(20 nmol/kg/天,皮下注射)治疗8周可逆转Morris水迷宫认知障碍(逃避潜伏期减少50%),降低海马TNF-α 60%,并改善胰岛素信号(p-AKT增加2倍)。 [4] |
| 酶活实验 |
与人GLP-1(7-36)NH2、GIP(1-42)、替西帕肽和赛马鲁肽的竞争结合基本上如同源竞争所述进行,不同之处在于测定缓冲液为1.0mM MgCl2、2.5mM CaCl2、0.003%w/v Tween-20、0.1%w/v杆菌肽在25mM HEPES中的最终浓度,pH 7.4,每50mL缓冲液加入一片完全不含EDTA的蛋白酶抑制剂片剂。使用GraphPad Prism 7软件,通过使用结合的量与添加的竞争同源肽的浓度的非线性回归分析来确定与GLP-1R和GIPR膜结合的[125I]GLP-1(7-36)NH2或[125I]GIP(1-42)的Bmax值。Bmax用于计算每个细胞的受体数量。对于竞争肽,Ki值通过非线性回归分析确定,使用结合的[125I]GLP-1(7-36)NH2或[125I]GIP(1-42)的量与所添加的肽的浓度。[2]
cAMP累积实验:表达人GIPR或GLP-1R的HEK293细胞经Tirzepatide(0.001-1000 nM)处理30分钟。使用均相时间分辨荧光(HTRF)试剂盒检测细胞内cAMP,剂量效应曲线计算EC₅₀。 [3] 受体结合动力学:用¹²⁵I标记的GIP或GLP-1进行放射配体结合实验。表达人受体的CHO-K1细胞膜与Tirzepatide(0.01-100 nM)孵育2小时,定量结合放射性以计算Ki。 [3] |
| 细胞实验 |
将稳定表达HA-GIPR-EFGP或HA–GLP-1R–EFGP克隆的HEK293细胞接种到聚-D-赖氨酸包被的96孔微孔板中,并培养直到细胞达到80%-90%的融合度。在测定当天,去除生长培养基,用预热的饥饿培养基(不含血清或抗生素的生长培养基、补充0.1%酪蛋白)冲洗细胞一次,并用新鲜培养基在37°C、5%CO2下平衡1小时。在预热的饥饿培养基中制备GLP-1、GIP和替西帕肽的浓度响应曲线,添加到细胞中指定时间,并在37°C下孵育。研究结束时,取出培养基,将细胞置于冰上,并用Prefer固定剂(Anatech)固定10分钟。去除固定剂,在PBS中洗涤细胞,并用Odyssey封闭缓冲液(Licor)封闭1小时。将细胞与抗HA/DyLight800抗体(1:700)(Rockland Immunocchemicals,600-445-384)孵育1小时,然后用PBS-T洗涤。使用带有800nm通道激光的Licor Clx扫描仪扫描板以捕获每个孔中的荧光信号。将数据标准化为GLP-1或GIP的最大浓度(100%)和无配体(0%),并通过非线性回归(S型浓度响应)进行分析,并使用GraphPad Prism 7软件绘制。[2]
|
| 动物实验 |
High fat diet and streptozotocin injection-induced diabetic rats were injected intraperitoneally with Tirzepatide (1.35 mg/kg) once a week. The protective effects were assessed using the Morris water maze test, immunofluorescence, and Western blot analysis. Golgi staining was adopted for quantified dendritic spines.[4]
Male Sprague Dawley rats weighing between 180 and 200 g (aged 7–8 weeks) were raised in Specific Pathogen Free (SPF) conditions with a light/dark cycle of 12 h/12 h and temperature–humidity (22°C ± 1°C, 50% ± 10%) controlled. All procedures were approved by the Animal Care and Use Committee of Hubei University of Science and Technology, Xianning, China (IACUC Number: 2021-03-003). Animal care and handling were performed according to the Declaration of management of laboratory animals regarding the care and use of laboratory animals. After 2 weeks adaptation with normal diet, a total of 32 rats were fed with HF diet (67.5% standard laboratory rat chow, 20% sugar, 10% lard, 2% cholesterol and 0.5% bile salts), while 24 rats were raised by standard chow. According to our previous study, 35 mg/kg STZ was injected by intraperitoneal injection in the rats of HF diet group, whereas normal group were injected with citrate buffer only. After 2 weeks feeding, 31 rats with a fasting blood glucose levels reaching 11.0 mmol/L were randomly divided into two experimental groups as follows: diabetes mellitus group (DM), DM + Tirzepatide group (Tirzepatide, 1.35 mg/kg, once a week). At the same time, 24 rats of standard chow group were randomly divided into control group (Con) and Con + Tirzepatide group (Tirzepatide, 1.35 mg/kg, once a week). All drugs were prepared preserving more than 1 year under given conditions avoiding degradation. Oral glucose tolerance test (OGTT) was performed on the 13th week. Behavioral test was conducted before the sacrificed week. Fasting blood glucose and body weight were measured weekly until the sacrificed week. In the 15th week, all rats were sacrificed and collected samples which were executed follow-up experiments. A timeline of experimental procedure is presented in Figure 1A.[4] db/db mice: Weekly SC injections of Tirzepatide (1, 3, 10 nmol/kg) or vehicle for 4 weeks. HbA1c and body weight monitored weekly. [3] Diabetic rat cognitive study: Streptozotocin-induced diabetic rats received daily SC Tirzepatide (20 nmol/kg) or saline for 8 weeks. Morris water maze tests performed at weeks 6-8; brains harvested for cytokine/AKT analysis. [4] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Over the dose range of 1-5 mg, the Cmax of tirzepatide ranged from 108 to 397 ng/mL. The mean absolute bioavailability of tirzepatide following subcutaneous administration is 80%. Following subcutaneous administration, the Tmax ranged from eight to 72 hours. The steady-state plasma concentrations were achieved following four weeks of once-weekly subcutaneous administration. As tirzepatide delays gastric emptying, it has the potential to affect the absorption of concomitantly administered oral medications. The US prescribing information recommends the use of caution when co-administering tirzepatide with other oral medications. Tirzepatide is primarily excreted via urine and feces, mostly in the form of metabolites. Unchanged parent drug was not detectable in urine and feces. Following subcutaneous administration, the mean steady-state volume of distribution was 9.5 L. The mean apparent steady-state volume of distribution of tirzepatide following subcutaneous administration in patients with type 2 diabetes mellitus was approximately 10.3 L. The apparent population mean clearance of tirzepatide is 0.061 L/h. The mean steady-state apparent clearance of tirzepatide was 0.056 L/h. Metabolism / Metabolites Tirzepatide is metabolized by proteolytic cleavage of the peptide backbone, beta-oxidation of the C20 fatty diacid moiety, and amide hydrolysis. Biological Half-Life The half-life is approximately five days. In humans, Tirzepatide has a half-life of ~5 days after SC administration. Steady-state concentrations achieved by 4 weeks with weekly dosing. [1] Plasma clearance: 0.061 L/h in T2D patients. [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In preregistration clinical trials, serum aminotransferase elevations of greater than 3 times the upper limit of normal (ULN) arose in less than 1% of patients during therapy with tirzepatide and similar rates occurred in placebo recipients and in comparator arm groups. In studies of more than 5,000 patients, there were no reports of severe liver test abnormalities or clinically apparent liver injury attributable to tirzepatide. However, tirzepatide has been associated with a slightly higher rate of acute gallbladder disease (cholelithiasis, biliary cholic and cholecystectomy) reported in 0.6% of treated patients vs none of placebo-treated patients. Gallbladder conditions are mentioned in the warning section of the product label for tirzepatide. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of tirzepatide during breastfeeding. Because tirzepatide is a large peptide molecule with a molecular weight of 4814 Da, the amount in milk is likely to be low and absorption is unlikely because it is probably partially destroyed in the infant's gastrointestinal tract. Until more data become available, tirzepatide should be used with caution during breastfeeding, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. ◈ What is tirzepatide? Tirzepatide is a medication that has been used to improve blood sugar control in adults with type 2 diabetes. It is available as an injection (given by shot). The injectable form is sold under the brand name Mounjaro®.Tirzepatide can also be used as an injection to treat obesity. A brand name for tirzepatide used for weight management is Zepbound®. Weight loss is not recommended during pregnancy. If you are using Zepound®, talk with your healthcare providers before making any changes to how you take your medication. Your healthcare providers can talk with you about the benefits of treating your condition and the risks of untreated illness during pregnancy.Obesity and elevated blood glucose can make it harder to get pregnant, and increase the chance of miscarriage, birth defects, or other pregnancy complications. MotherToBaby has fact sheets on diabetes https://mothertobaby.org/fact-sheets/type-1-and-type-2-diabetes/ and obesity https://mothertobaby.org/fact-sheets/obesity-pregnancy/.The product label for tirzepatide states the use of this medication might change the way oral contraceptives (birth control pills used to prevent pregnancy) are absorbed by the body. This might increase the chance of pregnancy, even if the oral birth control is taken correctly and consistently. The product label suggests people using oral contraceptives switch to a non-oral birth control or add a barrier method of contraception (like condoms) for 4 weeks after starting the medication and for 4 weeks after each increase in dose. If you are taking this medication, talk with your healthcare provider about non-oral birth control and all your options for preventing a pregnancy. ◈ I am taking tirzepatide, but I would like to stop taking it before getting pregnant. How long does the drug stay in my body? The time it takes to metabolize (break down) medication is not the same for everyone. In healthy adults, it can take up to 30 days, on average, for most of the tirzepatide to be gone from the body. ◈ I take tirzepatide. Can it make it harder for me to get pregnant? It is not known if tirzepatide can make it harder to get pregnant. ◈ Does taking tirzepatide increase the chance of miscarriage? Miscarriage is common and can occur in any pregnancy for many different reasons. Studies have not been done in humans to see if tirzepatide can increase the chance of miscarriage. ◈ Does taking tirzepatide increase the chance of birth defects? Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Research studies have not been done to see if tirzepatide increases the chance of birth defects in humans. In animal studies, an increased chance for some birth defects was seen. However, it is unclear if these birth defects were due to the medication or other factors in the study (such as weight loss). Diabetes with unmet glucose goals or targets in pregnancy can increase the chance of birth defects. It is important that diabetes is managed during pregnancy and glucose levels stay in your goal/target range throughout pregnancy. ◈ Does taking tirzepatide in pregnancy increase the chance of other pregnancy-related problems? Human studies have not been done to see if tirzepatide can increase the chance of pregnancy-related problems such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth). Animal studies reported a decrease in the weight of the offspring after exposure to tirzepatide in pregnancy. It is unclear if this was due to the medication, weight loss in the mother, or other factors. Diabetes with unmet glucose goals/targets in pregnancy can increase the chance of pregnancy complications. ◈ Does taking tirzepatide in pregnancy affect future behavior or learning for the child? Studies have not been done to see if tirzepatide can increase the chance of behavior or learning issues for the child. ◈ Breastfeeding while taking tirzepatide: There is no available information about tirzepatide and human milk. Because it is a large molecule, tirzepatide is not expected to get into breastmilk in large amounts. Also, the medication is likely to break down in the infant's gastrointestinal tract and not be well-absorbed by the infant. Be sure to talk to your healthcare provider about all your breastfeeding questions. ◈ If a male takes tirzepatide, could it affect fertility or increase the chance of birth defects? Studies have not been done in humans to see if tirzepatide could affect male fertility (ability to get partner pregnant) or increase the chance of birth defects above the background risk. There were no changes in male fertility reported in one animal study. In general, exposures that fathers or sperm donors have are unlikely to increase risks to a pregnancy. For more information, please see the MotherToBaby fact sheet Paternal Exposures at https://mothertobaby.org/fact-sheets/paternal-exposures-pregnancy/. Protein Binding Tirzepatide is 99% bound to plasma albumin. Common adverse events in humans: - Nausea (12-24%), diarrhea (12-18%), vomiting (6-10%) at 5-15 mg doses (mild-to-moderate severity) [1] No drug-related serious adverse events or deaths in phase 2 trial. [1] In diabetic rats, no behavioral abnormalities or organ toxicity observed at 20 nmol/kg/day. [4] |
| 参考文献 |
[1]. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018 Nov 17;392(10160):2180-2193.
[2]. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight. 2020 Sep 3; 5(17): e140532. [3]. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol Metab. 2018 Dec:18:3-14. [4]. Tirzepatide ameliorates spatial learning and memory impairment through modulation of aberrant insulin resistance and inflammation response in diabetic rats. Front Pharmacol. 2023 Aug 28;14:1146960. |
| 其他信息 |
Pharmacodynamics
Tirzepatide is a synthetic peptide with glucose-lowering effects. It works to stimulate first- and second-phase insulin secretion, and reduces glucagon levels, both in a glucose-dependent manner. Tirzepatide was also shown to delay gastric emptying, lower fasting and postprandial glucose concentration, decrease food intake, and reduce body weight in patients with type 2 diabetes. Tirzepatide can increase insulin sensitivity. As the peptide is conjugated to a C20 fatty diacid moiety through a hydrophilic linker at the lysine residue at position 20, the drug is highly bound to albumin in the plasma, which prolongs its half-life. Background: LY3298176 is a novel dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that is being developed for the treatment of type 2 diabetes. We aimed to examine the efficacy and safety of co-stimulation of the GLP-1 and GIP receptors with LY3298176 compared with placebo or selective stimulation of GLP-1 receptors with dulaglutide in patients with poorly controlled type 2 diabetes. Methods: In this double-blind, randomised, phase 2 study, patients with type 2 diabetes were randomly assigned (1:1:1:1:1:1) to receive either once-weekly subcutaneous LY3298176 (1 mg, 5 mg, 10 mg, or 15 mg), dulaglutide (1·5 mg), or placebo for 26 weeks. Assignment was stratified by baseline glycated haemoglobin A1c (HbA1c), metformin use, and body-mass index (BMI). Eligible participants (aged 18-75) had type 2 diabetes for at least 6 months (HbA1c 7·0-10·5%, inclusive), that was inadequately controlled with diet and exercise alone or with stable metformin therapy, and a BMI of 23-50 kg/m2. The primary efficacy outcome was change in HbA1c from baseline to 26 weeks in the modified intention-to-treat (mITT) population (all patients who received at least one dose of study drug and had at least one postbaseline measurement of any outcome). Secondary endpoints, measured in the mITT on treatment dataset, were change in HbA1c from baseline to 12 weeks; change in mean bodyweight, fasting plasma glucose, waist circumference, total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides, and proportion of patients reaching the HbA1c target (≤6·5% and <7·0%) from baseline to weeks 12 and 26; and proportion of patients with at least 5% and 10% bodyweight loss from baseline to 26 weeks. This study is registered with ClinicalTrials.gov, number NCT03131687. Findings: Between May 24, 2017, and March 28, 2018, 555 participants were assessed for eligibility, of whom 318 were randomly assigned to one of the six treatment groups. Because two participants did not receive treatment, the modified intention-to-treat and safety populations included 316 participants. 258 (81·7%) participants completed 26 weeks of treatment, and 283 (89·6%) completed the study. At baseline, mean age was 57 years (SD 9), BMI was 32·6 kg/m2 (5·9), duration from diagnosis of diabetes was 9 years (6), HbA1c was 8·1% (1·0), 53% of patients were men, and 47% were women. At 26 weeks, the effect of LY3298176 on change in HbA1c was dose-dependent and did not plateau. Mean changes from baseline in HbA1c with LY3298176 were -1·06% for 1 mg, -1·73% for 5 mg, -1·89% for 10 mg, and -1·94% for 15 mg, compared with -0·06% for placebo (posterior mean differences [80% credible set] vs placebo: -1·00% [-1·22 to -0·79] for 1 mg, -1·67% [-1·88 to -1·46] for 5 mg, -1·83% [-2·04 to -1·61] for 10 mg, and -1·89% [-2·11 to -1·67] for 15 mg). Compared with dulaglutide (-1·21%) the posterior mean differences (80% credible set) for change in HbA1c from baseline to 26 weeks with the LY3298176 doses were 0·15% (-0·08 to 0·38) for 1 mg, -0·52% (-0·72 to -0·31) for 5 mg, -0·67% (-0·89 to -0·46) for 10 mg, and -0·73% (-0·95 to -0·52) for 15 mg. At 26 weeks, 33-90% of patients treated with LY3298176 achieved the HbA1c target of less than 7·0% (vs 52% with dulaglutide, 12% with placebo) and 15-82% achieved the HbA1c target of at least 6·5% (vs 39% with dulaglutide, 2% with placebo). Changes in fasting plasma glucose ranged from -0·4 mmol/L to -3·4 mmol/L for LY3298176 (vs 0·9 mmol/L for placebo, -1·2 mmol/L for dulaglutide). Changes in mean bodyweight ranged from -0·9 kg to -11·3 kg for LY3298176 (vs -0·4 kg for placebo, -2·7 kg for dulaglutide). At 26 weeks, 14-71% of those treated with LY3298176 achieved the weight loss target of at least 5% (vs 22% with dulaglutide, 0% with placebo) and 6-39% achieved the weight loss target of at least 10% (vs 9% with dulaglutide, 0% with placebo). Changes in waist circumference ranged from -2·1 cm to -10·2 cm for LY3298176 (vs -1·3 cm for placebo, -2·5 cm for dulaglutide). Changes in total cholesterol ranged from 0·2 mmol/L to -0·3 mmol/L for LY3298176 (vs 0·3 mmol/L for placebo, -0·2 mmol/L for dulaglutide). Changes in HDL or LDL cholesterol did not differ between the LY3298176 and placebo groups. Changes in triglyceride concentration ranged from 0 mmol/L to -0·8 mmol/L for LY3298176 (vs 0·3 mmol/L for placebo, -0·3 mmol/L for dulaglutide). The 12-week outcomes were similar to those at 26 weeks for all secondary outcomes. 13 (4%) of 316 participants across the six treatment groups had 23 serious adverse events in total. Gastrointestinal events (nausea, diarrhoea, and vomiting) were the most common treatment-emergent adverse events. The incidence of gastrointestinal events was dose-related (23·1% for 1 mg LY3298176, 32·7% for 5 mg LY3298176, 51·0% for 10 mg LY3298176, and 66·0% for 15 mg LY3298176, 42·6% for dulaglutide, 9·8% for placebo); most events were mild to moderate in intensity and transient. Decreased appetite was the second most common adverse event (3·8% for 1 mg LY3298176, 20·0% for 5 mg LY3298176, 25·5% for 10 mg LY3298176, 18·9% for 15 mg LY3298176, 5·6% for dulaglutide, 2·0% for placebo). There were no reports of severe hypoglycaemia. One patient in the placebo group died from lung adenocarcinoma stage IV, which was unrelated to study treatment. Interpretation: The dual GIP and GLP-1 receptor agonist, LY3298176, showed significantly better efficacy with regard to glucose control and weight loss than did dulaglutide, with an acceptable safety and tolerability profile. Combined GIP and GLP-1 receptor stimulation might offer a new therapeutic option in the treatment of type 2 diabetes. [1] Tirzepatide (LY3298176) is a dual GIP and GLP-1 receptor agonist under development for the treatment of type 2 diabetes mellitus (T2DM), obesity, and nonalcoholic steatohepatitis. Early phase trials in T2DM indicate that tirzepatide improves clinical outcomes beyond those achieved by a selective GLP-1 receptor agonist. Therefore, we hypothesized that the integrated potency and signaling properties of tirzepatide provide a unique pharmacological profile tailored for improving broad metabolic control. Here, we establish methodology for calculating occupancy of each receptor for clinically efficacious doses of the drug. This analysis reveals a greater degree of engagement of tirzepatide for the GIP receptor than the GLP-1 receptor, corroborating an imbalanced mechanism of action. Pharmacologically, signaling studies demonstrate that tirzepatide mimics the actions of native GIP at the GIP receptor but shows bias at the GLP-1 receptor to favor cAMP generation over β-arrestin recruitment, coincident with a weaker ability to drive GLP-1 receptor internalization compared with GLP-1. Experiments in primary islets reveal β-arrestin1 limits the insulin response to GLP-1, but not GIP or tirzepatide, suggesting that the biased agonism of tirzepatide enhances insulin secretion. Imbalance toward GIP receptor, combined with distinct signaling properties at the GLP-1 receptor, together may account for the promising efficacy of this investigational agent. [2] Objective: A novel dual GIP and GLP-1 receptor agonist, LY3298176, was developed to determine whether the metabolic action of GIP adds to the established clinical benefits of selective GLP-1 receptor agonists in type 2 diabetes mellitus (T2DM). Methods: LY3298176 is a fatty acid modified peptide with dual GIP and GLP-1 receptor agonist activity designed for once-weekly subcutaneous administration. LY3298176 was characterised in vitro, using signaling and functional assays in cell lines expressing recombinant or endogenous incretin receptors, and in vivo using body weight, food intake, insulin secretion and glycemic profiles in mice. A Phase 1, randomised, placebo-controlled, double-blind study was comprised of three parts: a single-ascending dose (SAD; doses 0.25-8 mg) and 4-week multiple-ascending dose (MAD; doses 0.5-10 mg) studies in healthy subjects (HS), followed by a 4-week multiple-dose Phase 1 b proof-of-concept (POC; doses 0.5-15 mg) in patients with T2DM (ClinicalTrials.gov no. NCT02759107). Doses higher than 5 mg were attained by titration, dulaglutide (DU) was used as a positive control. The primary objective was to investigate safety and tolerability of LY3298176. Results: LY3298176 activated both GIP and GLP-1 receptor signaling in vitro and showed glucose-dependent insulin secretion and improved glucose tolerance by acting on both GIP and GLP-1 receptors in mice. With chronic administration to mice, LY3298176 potently decreased body weight and food intake; these effects were significantly greater than the effects of a GLP-1 receptor agonist. A total of 142 human subjects received at least 1 dose of LY3298176, dulaglutide, or placebo. The PK profile of LY3298176 was investigated over a wide dose range (0.25-15 mg) and supports once-weekly administration. In the Phase 1 b trial of diabetic subjects, LY3298176 doses of 10 mg and 15 mg significantly reduced fasting serum glucose compared to placebo (least square mean [LSM] difference [95% CI]: -49.12 mg/dL [-78.14, -20.12] and -43.15 mg/dL [-73.06, -13.21], respectively). Reductions in body weight were significantly greater with the LY3298176 1.5 mg, 4.5 mg and 10 mg doses versus placebo in MAD HS (LSM difference [95% CI]: -1.75 kg [-3.38, -0.12], -5.09 kg [-6.72, -3.46] and -4.61 kg [-6.21, -3.01], respectively) and doses of 10 mg and 15 mg had a relevant effect in T2DM patients (LSM difference [95% CI]: -2.62 kg [-3.79, -1.45] and -2.07 kg [-3.25, -0.88], respectively. The most frequent side effects reported with LY3298176 were gastrointestinal (vomiting, nausea, decreased appetite, diarrhoea, and abdominal distension) in both HS and patients with T2DM; all were dose-dependent and considered mild to moderate in severity. Conclusions: Based on these results, the pharmacology of LY3298176 translates from preclinical to clinical studies. LY3298176 has the potential to deliver clinically meaningful improvement in glycaemic control and body weight. The data warrant further clinical evaluation of LY3298176 for the treatment of T2DM and potentially obesity. [3] |
| 分子式 |
C225H348N48O68
|
|---|---|
| 分子量 |
4813.45147800446
|
| 精确质量 |
4810.52
|
| 元素分析 |
C, 56.14; H, 7.29; N, 13.97; O, 22.60
|
| CAS号 |
2023788-19-2
|
| 相关CAS号 |
Tirzepatide hydrochloride; Tirzepatide TFA;13C,15N Tirzepatide;Tirzepatide TFA (LY3298176 TFA);Tirzepatide hydrochloride (LY3298176 hydrochloride); 2933217-72-0 (sodium salt); 2931515-08-9 (acetate)
|
| PubChem CID |
168009818
|
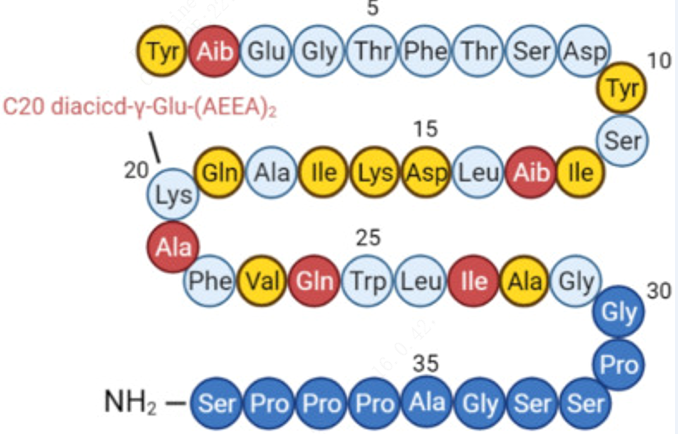
| 序列 |
Tyr-{Aib}-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Ile-{Aib}-Leu-Asp-Lys-Ile-Ala-Gln-{C20 diacid-gamma-Glu-(AEEA)2-Lys}-Ala-Phe-Val-Gln-Trp-Leu-Ile-Ala-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-NH2
|
| 短序列 |
Y-{Aib}-EGTFTSDYSI-{Aib}-LDKIAQ-{C20 diacid-gamma-Glu-(AEEA)2-Lys}-AFVQWLIAGGPSSGAPPPS-NH2;
or
YXEGTFTSDY SIXLDKIAQK AFVQWLIAGG PSSGAPPPS
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
95.0~105.0%
|
| LogP |
-6.8
|
| tPSA |
1790Ų
|
| 氢键供体(HBD)数目 |
58
|
| 氢键受体(HBA)数目 |
70
|
| 可旋转键数目(RBC) |
163
|
| 重原子数目 |
341
|
| 分子复杂度/Complexity |
11700
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCCCNC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](C(=O)O)NC(=O)CCCCCCCCCCCCCCCCCCC(=O)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)NCC(=O)NCC(=O)N4CCC[C@H]4C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C)C(=O)N5CCC[C@H]5C(=O)N6CCC[C@H]6C(=O)N7CCC[C@H]7C(=O)N[C@@H](CO)C(=O)N)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)C(C)(C)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@H](CO)NC(=O)[C@H](CC8=CC=C(C=C8)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC9=CC=CC=C9)NC(=O)[C@H]([C@@H](C)O)NC(=O)CNC(=O)[C@H](CCC(=O)O)NC(=O)C(C)(C)NC(=O)[C@H](CC1=CC=C(C=C1)O)N.CC(=O)O
|
| InChi Key |
BTSOGEDATSQOAF-SMAAHMJQSA-N
|
| InChi Code |
InChI=1S/C225H348N48O68/c1-23-126(10)183(264-198(311)146(64-50-52-88-226)246-202(315)157(109-180(297)298)252-199(312)152(103-124(6)7)261-223(337)225(21,22)269-217(330)185(128(12)25-3)266-209(322)163(120-278)257-200(313)153(107-138-74-78-141(282)79-75-138)250-203(316)158(110-181(299)300)253-207(320)162(119-277)259-216(329)187(134(18)280)267-206(319)155(106-136-60-44-41-45-61-136)254-215(328)186(133(17)279)262-174(289)114-237-193(306)147(83-87-179(295)296)260-222(336)224(19,20)268-192(305)143(227)104-137-72-76-140(281)77-73-137)214(327)242-131(15)190(303)244-148(80-84-168(228)283)196(309)245-145(65-51-53-89-231-175(290)121-340-100-99-339-97-91-233-176(291)122-341-101-98-338-96-90-232-170(285)86-82-150(221(334)335)243-171(286)70-46-38-36-34-32-30-28-26-27-29-31-33-35-37-39-47-71-178(293)294)195(308)240-130(14)191(304)248-154(105-135-58-42-40-43-59-135)205(318)263-182(125(8)9)212(325)247-149(81-85-169(229)284)197(310)251-156(108-139-111-234-144-63-49-48-62-142(139)144)201(314)249-151(102-123(4)5)204(317)265-184(127(11)24-2)213(326)241-129(13)189(302)236-112-172(287)235-115-177(292)270-92-54-66-164(270)210(323)258-161(118-276)208(321)256-160(117-275)194(307)238-113-173(288)239-132(16)218(331)272-94-56-68-166(272)220(333)273-95-57-69-167(273)219(332)271-93-55-67-165(271)211(324)255-159(116-274)188(230)301/h40-45,48-49,58-63,72-79,111,123-134,143,145-167,182-187,234,274-282H,23-39,46-47,50-57,64-71,80-110,112-122,226-227H2,1-22H3,(H2,228,283)(H2,229,284)(H2,230,301)(H,231,290)(H,232,285)(H,233,291)(H,235,287)(H,236,302)(H,237,306)(H,238,307)(H,239,288)(H,240,308)(H,241,326)(H,242,327)(H,243,286)(H,244,303)(H,245,309)(H,246,315)(H,247,325)(H,248,304)(H,249,314)(H,250,316)(H,251,310)(H,252,312)(H,253,320)(H,254,328)(H,255,324)(H,256,321)(H,257,313)(H,258,323)(H,259,329)(H,260,336)(H,261,337)(H,262,289)(H,263,318)(H,264,311)(H,265,317)(H,266,322)(H,267,319)(H,268,305)(H,269,330)(H,293,294)(H,295,296)(H,297,298)(H,299,300)(H,334,335)/t126-,127-,128-,129-,130-,131-,132-,133+,134+,143-,145-,146-,147-,148-,149-,150+,151-,152-,153-,154-,155-,156-,157-,158-,159-,160-,161-,162-,163-,164-,165-,166-,167-,182-,183-,184-,185-,186-,187-/m0/s1
|
| 化学名 |
20-[[(1R)-4-[2-[2-[2-[2-[2-[2-[[(5S)-5-[[(2S)-5-amino-2-[[(2S)-2-[[(2S,3S)-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[2-[[(2S,3S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,3R)-2-[[(2S)-2-[[(2S,3R)-2-[[2-[[(2S)-2-[[2-[[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]amino]-2-methylpropanoyl]amino]-4-carboxybutanoyl]amino]acetyl]amino]-3-hydroxybutanoyl]amino]-3-phenylpropanoyl]amino]-3-hydroxybutanoyl]amino]-3-hydroxypropanoyl]amino]-3-carboxypropanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-hydroxypropanoyl]amino]-3-methylpentanoyl]amino]-2-methylpropanoyl]amino]-4-methylpentanoyl]amino]-3-carboxypropanoyl]amino]hexanoyl]amino]-3-methylpentanoyl]amino]propanoyl]amino]-5-oxopentanoyl]amino]-6-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-5-amino-1-[[(2S)-1-[[(2S)-1-[[(2S,3S)-1-[[(2S)-1-[[2-[[2-[(2S)-2-[[(2S)-1-[[(2S)-1-[[2-[[(2S)-1-[(2S)-2-[(2S)-2-[(2S)-2-[[(2S)-1-amino-3-hydroxy-1-oxopropan-2-yl]carbamoyl]pyrrolidine-1-carbonyl]pyrrolidine-1-carbonyl]pyrrolidin-1-yl]-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]carbamoyl]pyrrolidin-1-yl]-2-oxoethyl]amino]-2-oxoethyl]amino]-1-oxopropan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-1-oxopropan-2-yl]amino]-6-oxohexyl]amino]-2-oxoethoxy]ethoxy]ethylamino]-2-oxoethoxy]ethoxy]ethylamino]-1-carboxy-4-oxobutyl]amino]-20-oxoicosanoic acid
|
| 别名 |
LY-3298176; LY 3298176; tirzepatide; LY3298176; BG 121; BG121; BG-121
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 1) 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 2) 该产品在溶液状态不稳定,请现配现用。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~50 mg/mL (~10.4 mM)
Ethanol : 8~9 mg/mL Water : Insoluble |
|---|---|
| 溶解度 (体内实验) |
Note: 如何溶解多肽产品?请参考本产品网页右上角“产品说明书“文件,第4页。 注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。 注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.2078 mL | 1.0388 mL | 2.0775 mL | |
| 5 mM | 0.0416 mL | 0.2078 mL | 0.4155 mL | |
| 10 mM | 0.0208 mL | 0.1039 mL | 0.2078 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Research Study to See How Much CagriSema (1.0 mg Once Weekly) Lowers Blood Sugar and Body Weight Compared to Tirzepatide (5 mg Once Weekly) in People With Type 2 Diabetes Treated With Metformin, SGLT2 Inhibitor or Both
CTID: NCT06534411
Phase: Phase 3 Status: R
A Randomized, Double-Blind, Placebo-Controlled Phase 2 Study Comparing
CTID: null
Phase: Phase 2 Status: Ongoing, GB - no longer in EU/EEA, Completed
Date: 2020-01-23