| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|

| 体外研究 (In Vitro) |
5-羟色胺转运蛋白放射性配体 [3H]-帕罗西汀与转染人 5-HT 转运蛋白的细胞膜的结合受到文拉法辛 (Wy 45030) 的剂量依赖性抑制,Ki 为 2.48 μM。文拉法辛的 Ki 值为 82 nM,可防止 NE 转运蛋白配体 [3H]-nisoxetine 附着在转染的人 NE 转运蛋白的膜上 [1]。文拉法辛的 ED50 值分别为 2 和 54 mg/kg,在体外抑制与大鼠 5-HT 转运蛋白和 NE 转运蛋白的结合[1]。
|
|---|---|
| 体内研究 (In Vivo) |
在大鼠下丘脑中,文拉法辛(Wy 45030;10-100 mg/kg;IP)剂量依赖性地阻止 6-OHDA 诱导的去甲肾上腺素水平降低 [1]。
|
| 动物实验 |
Animal/Disease Models: Male SD (SD (Sprague-Dawley)) rats, body weight 180-230 grams [1]
Doses: 10, 30, 100 mg/kg Route of Administration: IP; para-chloramphetamine hydrochloride (p-CA; 10 mg/kg; intraperitoneal (ip) injection ) Results one hour before: dose-dependently blocked 6-OHDA-induced depletion of norepinephrine levels in the rat hypothalamus (intracerebroventricular; 50 μg/rat; one hour later)), ED50 values were 12 and 94 mg/kg. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Venlafaxine is well absorbed after oral administration with an absolute bioavailability of approximately 45%. In mass balance studies, at least 92% of a single oral dose of venlafaxine was absorbed. After twice-daily oral administration of immediate-release formulation of 150 mg venlafaxine, Cmax was 150 ng/mL and Tmax was 5.5 hours. Cmax and Tmax of ODV were 260 ng/mL and nine hours, respectively. The extended-release formulation of venlafaxine has a slower rate of absorption, but the same extent of absorption as the immediate-release formulation. After once-daily administration of extended-release formulation of 75 mg venlafaxine, Cmax was 225 ng/mL and Tmax was two hours. Cmax and Tmax of ODV were 290 ng/mL and three hours, respectively. Food does not affect the bioavailability of venlafaxine or its active metabolite, O-desmethylvenlafaxine (ODV). Approximately 87% of a venlafaxine dose is recovered in the urine within 48 hours as unchanged venlafaxine (5%), unconjugated ODV (29%), conjugated ODV (26%), or other minor inactive metabolites (27%). The apparent volume of distribution at steady-state is 7.5 ± 3.7 L/kg for venlafaxine and 5.7 ± 1.8 L/kg for ODV. Mean ± SD plasma apparent clearance at steady-state is 1.3 ± 0.6 L/h/kg for venlafaxine and 0.4 ± 0.2 L/h/kg for ODV. Venlafaxine is well absorbed ... .On the basis of mass balance studies, at least 92% of a single oral dose of venlafaxine is absorbed. The absolute bioavailability of venlafaxine is about 45% Steady-state concentrations of venlafaxine and O-desmethylvenlafaxine in plasma are attained within 3 days of oral multiple dose therapy. Venlafaxine and O-desmethylvenlafaxine exhibited linear kinetics over the dose range of 75 to 450 mg/day. Mean +/-SD steady-state plasma clearance of venlafaxine and O-desmethylvenlafaxine is 1.3 +/- 0.6 and 0.4 0.2 L/hr/kg, respectively; apparent elimination half-life is 5 +/- 2 and 11 +/- 2 hours, respectively; and apparent (steady-state) volume of distribution is 7.5 +/- 3.7 and 5.7 +/- 1.8 L/kg, respectively. Venlafaxine and O-desmethylvenlafaxine are minimally bound at therapeutic concentrations to plasma proteins (27% and 30%, respectively). Approximately 87% of a venlafaxine dose is recovered in the urine within 48 hours as unchanged venlafaxine (5%), unconjugated O-desmethylvenlafaxine (29%), conjugated O-desmethylvenlafaxine (26%), or other minor inactive metabolites (27%). Renal elimination of venlafaxine and its metabolites is thus the primary route of excretion Venlafaxine is a unique antidepressant ... . The pharmacokinetics and relative bioavailability of venlafaxine were evaluated in healthy volunteers after oral administration. The bioavailability of 50 mg of venlafaxine as a tablet relative to a solution was determined in a two-period randomized crossover study. The rate of absorption from the gastrointestinal tract was assessed by the time to peak plasma concentration (tmax), a model-dependent calculation of the first-order absorption rate constant, and a model-independent calculation of mean residence time. The extent of absorption was assessed by peak plasma concentration (Cmax) and area under the concentration-time curve (AUC). No statistically significant differences were observed between the two formulations for either the rate or extent of absorption. Similarly, systemic concentrations of the active O-demethylated metabolite did not significantly differ after administration of the two venlafaxine formulations. AUC ratios indicated that the relative bioavailabilities of the parent drug, and formulation of metabolite were approximately 98% and 92%, respectively, for the tablet versus the solution. A separate study was conducted to examine the influence of food on venlafaxine absorption from the 50-mg tablet. A standard, medium-fat breakfast eaten immediately before drug administration delayed the tmax of venlafaxine but did not affect Cmax or AUC. Therefore the tablet formulation of venlafaxine is bioequivalent to the oral solution, and the presence of food appears to decrease the rate but not the extent of absorption of venlafaxine from the tablet formulation. For more Absorption, Distribution and Excretion (Complete) data for Venlafaxine (8 total), please visit the HSDB record page. Metabolism / Metabolites Following absorption, venlafaxine undergoes extensive presystemic metabolism in the liver. It primarily undergoes CYP2D6-mediated demethylation to form its active metabolite O-desmethylvenlafaxine (ODV). Venlafaxine can also undergo N-demethylation mediated by CYP2C9, and CYP2C19, and CYP3A4 to form N-desmethylvenlafaxine (NDV) but this is a minor metabolic pathway. ODV and NDV further metabolized by CYP2C19, CYP2D6 and/or CYP3A4 to form N,O-didesmethylvenlafaxine (NODV) and NODV can be further metabolized to form N, N, O-tridesmethylvenlafaxine, followed by a possible glucuronidation. Following absorption, venlafaxine undergoes extensive presystemic metabolism in the liver, primarily to O-desmethylvenlafaxine, but also to N-desmethylvenlafaxine, N,O-didesmethylvenlafaxine, and other minor metabolites. In vitro studies indicate that the formation of O-desmethylvenlafaxine is catalyzed by CYP2D6; this has been confirmed in a clinical study showing that patients with low CYP2D6 levels ("poor metabolizers") had increased levels of venlafaxine and reduced levels of O-desmethylvenlafaxine compared to people with normal CYP2D6 ("extensive metabolizers"). The differences between the CYP2D6 poor and extensive metabolizers, however, are not expected to be clinically important because the sum of venlafaxine and O-desmethylvenlafaxine is similar in the two groups and venlafaxine and O-desmethylvenlafaxine are pharmacologically approximately equiactive and equipotent. The biotransformation of venlafaxine (VF) into its two major metabolites, O-desmethylvenlafaxine (ODV) and N-desmethylvenlafaxine (NDV) was studied in vitro with human liver microsomes and with microsomes containing individual human cytochromes from cDNA-transfected human lymphoblastoid cells. VF was coincubated with selective cytochrome P450 (CYP) inhibitors and several selective serotonin reuptake inhibitors (SSRIs) to assess their inhibitory effect on VF metabolism. Formation rates for ODV incubated with human microsomes were consistent with Michaelis-Menten kinetics for a single-enzyme mediated reaction with substrate inhibition. Mean parameters determined by non-linear regression were: Vmax = 0.36 nmol/min/mg protein, K(m) = 41 microM, and Ks 22901 microM (Ks represents a constant which reflects the degree of substrate inhibition). Quinidine (QUI) was a potent inhibitor of ODV formation with a Ki of 0.04 microM, and paroxetine (PX) was the most potent SSRI at inhibiting ODV formation with a mean Ki value of 0.17 microM. Studies using expressed cytochromes showed that ODV was formed by CYP2C9, -2C19, and -2D6. CYP2D6 was dominant with the lowest K(m), 23.2 microM, and highest intrinsic clearance (Vmax/K(m) ratio). No unique model was applicable to the formation of NDV for all four livers tested. Parameters determined by applying a single-enzyme model were Vmax = 2.14 nmol/min/mg protein, and K(m) = 2504 microM. Ketoconazole was a potent inhibitor of NDV production, although its inhibitory activity was not as great as observed with pure 3A substrates. NDV formation was also reduced by 42% by a polyclonal rabbit antibody against rat liver CYP3A1. Studies using expressed cytochromes showed that NDV was formed by CYP2C9, -2C19, and -3A4. The highest intrinsic clearance was attributable to CYP2C19 and the lowest to CYP3A4. However the high in vivo abundance of 3A isoforms will magnify the importance of this cytochrome. Fluvoxamine (FX), at a concentration of 20 microM, decreased NDV production by 46% consistent with the capacity of FX to inhibit CYP3A, 2C9, and 2C19. These results are consistent with previous studies that show CYP2D6 and -3A4 play important roles in the formation of ODV and NDV, respectively. In addition we have shown that several other CYPs have important roles in the biotransformation of VF. On three occasions, unusually high trough plasma concentrations of venlafaxine were measured in a patient phenotyped and genotyped as being an extensive CYP2D6 metabolizer and receiving 450 mg/day of venlafaxine and multiple comedications. Values of 1.54 and of 0.60 mg/l of venlafaxine and O-desmethylvenlafaxine, respectively, were determined in the first blood sample, giving an unusually high venlafaxine to O-desmethylvenlafaxine ratio. This suggests an impaired metabolism of venlafaxine to O-desmethylvenlafaxine, and is most likely due to metabolic interactions with mianserin (240 mg/day) and propranolol (40 mg/day). Concentration of (S)-venlafaxine measured in this blood sample was almost twice as high as (R)-venlafaxine ((S)/(R) ratio: 1.94). At the second blood sampling, after addition of thioridazine (260 mg/day), which is a strong CYP2D6 inhibitor, concentrations of venlafaxine were further increased (2.76 mg/l), and concentrations of O-desmethylvenlafaxine decreased (0.22 mg/l). A decrease of the (S)/(R)-venlafaxine ratio (-20%) suggests a possible stereoselectivity towards the (R)-enantiomer of the enzyme(s) involved in venlafaxine O-demethylation at these high venlafaxine concentrations. At the third blood sampling, after interruption of thioridazine, concentrations of venlafaxine and O-desmethylvenlafaxine were similar to those measured in the first blood sample. This case report shows the importance of performing studies on the effects of either genetically determined or acquired deficiency of metabolism on the kinetics of venlafaxine. Approximately 87% of a venlafaxine dose is recovered in the urine within 48 hours as unchanged venlafaxine (5%), unconjugated O-desmethylvenlafaxine (29%), conjugated O-desmethylvenlafaxine (26%), or other minor inactive metabolites (27%). Renal elimination of venlafaxine and its metabolites is thus the primary route of excretion Undergoes extensive first pass metabolism in the liver to its major, active metabolite, ODV, and two minor, less active metabolites, N-desmethylvenlafaxine and N,O-didesmethylvenlafaxine. Formation of ODV is catalyzed by cytochrome P450 (CYP) 2D6, whereas N-demethylation is catalyzed by CYP3A4, 2C19 and 2C9. ODV possesses antidepressant activity that is comparable to that of venlfaxine. Route of Elimination: Renal elimination of venlafaxine and its metabolites is the primary route of excretion. Approximately 87% of a venlafaxine dose is recovered in the urine within 48 hours as either unchanged venlafaxine (5%), unconjugated ODV (29%), conjugated ODV (26%), or other minor inactive metabolites (27%). Half Life: 5 hours Biological Half-Life The apparent elimination half-life is 5 ± 2 hours for venlafaxine and 11 ± 2 hours for ODV. Apparent elimination half-life /of venlafaxine and O-desmethylvenlafaxine/ is 5 +/- 2 and 11 +/- 2 hours, respectively. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
The exact mechanism of action of venlafaxine is unknown, but appears to be associated with the its potentiation of neurotrasmitter activity in the CNS. Venlafaxine and its active metabolite, O-desmethylvenlafaxine (ODV), inhibit the reuptake of both serotonin and norepinephrine with a potency greater for the 5-HT than for the NE reuptake process. Both venlafaxine and the ODV metabolite have weak inhibitory effects on the reuptake of dopamine but, unlike the tricyclics and similar to SSRIs, they are not active at histaminergic, muscarinic, or alpha(1)-adrenergic receptors. Interactions Although venlafaxine has not been shown to increase the impairment of mental and motor skills caused by alcohol, patients should be advised to avoid alcohol while taking venlafaxine. A 25-year-old white woman with chronic depression was treated with venlafaxine 150 mg/day and trimipramine 50 mg/day. Eleven days after increase of the trimipramine dosage to 100 mg/d, she was hospitalized because of seizures suggesting a secondary generalized grand-mal episode. The electroencephalogram showed a pathologic pattern with several generalized epileptiform discharges. Because of suspected drug-induced seizures, both antidepressants were stopped. After antidepressant drug cessation, the patient was symptom free and had no further seizure episodes within the following 12 months of follow-up. No other potential cause for the seizure episode could be identified. Both venlafaxine and trimipramine have been associated with seizures, mainly after overdose. Venlafaxine-associated seizures at therapeutic doses have not been reported in the literature. /It was/ hypothesize that a pharmacodynamic or pharmacokinetic drug interaction between venlafaxine and trimipramine involving the CYP2D6 isoenzyme may have played a role in inducing the seizures. A patient developed neuroleptic malignant syndrome after a single dose of venlafaxine with trifluoperazine treatment. A dopamine-inhibition effect induced by one dose of venlafaxine may have augmented dopamine-receptor inhibition by trifluoperazine. Concomitant administration of cimetidine and venlafaxine in a steady-state study for both drugs resulted in inhibition of first-pass metabolism of venlafaxine in 18 healthy subjects. The oral clearance of venlafaxine was reduced by about 43%, and the exposure (AUC) and maximum concentration (Cmax) of the drug were increased by about 60%. However, coadministration of cimetidine had no apparent effect on the pharmacokinetics of O-desmethylvenlafaxine, which is present in much greater quantity in the circulation than venlafaxine. The overall pharmacological activity of venlafaxine plus O-desmethylvenlafaxine is expected to increase only slightly, and no dosage adjustment should be necessary for most normal adults. However, for patients with pre-existing hypertension, and for elderly patients or patients with hepatic dysfunction, the interaction associated with the concomitant use of venlafaxine and cimetidine is not known and potentially could be more pronounced. Therefore, caution is advised with such patients. For more Interactions (Complete) data for Venlafaxine (24 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Antidepressive Agents, Second-Generation; Serotonin Uptake Inhibitors Venlafaxine hydrochloride is used in the treatment of major depressive disorder. /Included in US product labeling/ Venlafaxine hydrochloride is used in the treatment of generalized anxiety disorder. /Included in US product labeling/ Venlafaxine hydrochloride is used in the treatment of social phobia (social anxiety disorder). /Included in US product labeling/ For more Therapeutic Uses (Complete) data for Venlafaxine (10 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ WARNING: SUICIDAL THOUGHTS AND BEHAVIORS. Antidepressants increased the risk of suicidal thoughts and behavior in children, adolescents, and young adults in short-term studies. These studies did not show an increase in the risk of suicidal thoughts and behavior with antidepressant use in patients over age 24; there was a reduction in risk with antidepressant use in patients aged 65 and older. In patients of all ages who are started on antidepressant therapy monitor closely for clinical worsening and emergence of suicidal thoughts and behaviors. Advise families and caregivers of the need for close observation and communication with the prescriber. The US Food and Drug Administration (FDA) recommends that all patients being treated with antidepressants for any indication be appropriately monitored and closely observed for clinical worsening, suicidality, and unusual changes in behavior, particularly during initiation of therapy (i.e., the first few months) and during periods of dosage adjustments. Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be advised to monitor patients on a daily basis for the emergence of agitation, irritability, or unusual changes in behavior, as well as the emergence of suicidality, and to report such symptoms immediately to a health-care provider. Although a causal relationship between the emergence of symptoms such as anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia, hypomania, and/or mania and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality. Consequently, consideration should be given to changing the therapeutic regimen or discontinuing therapy in patients whose depression is persistently worse or in patients experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, particularly if such manifestations are severe, abrupt in onset, or were not part of the patient's presenting symptoms. If a decision is made to discontinue therapy, venlafaxine dosage should be tapered as rapidly as is feasible but with recognition of the risks of abrupt discontinuance. A case of venlafaxine-induced serotonin syndrome is described with relapse following the introduction of amitriptyline, despite a 2-week period between the discontinuation of one drug and the commencement of the other. Electroencephalography may play an important part in diagnosis. With the increasing use of selective serotonin re-uptake inhibitors, greater awareness of the serotonin syndrome is necessary. Furthermore, the potential for drug interactions which may lead to the syndrome needs to be recognized. For more Drug Warnings (Complete) data for Venlafaxine (20 total), please visit the HSDB record page. Pharmacodynamics Venlafaxine is an antidepressant agent that works to ameliorate the symptoms of various psychiatric disorders by increasing the level of neurotransmitters in the synapse. Venlafaxine does not mediate muscarinic, histaminergic, or adrenergic effects. |
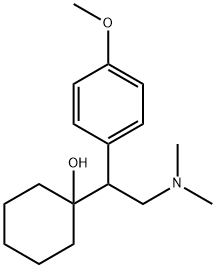
| 分子式 |
C17H27NO2
|
|---|---|
| 分子量 |
277.40178
|
| 精确质量 |
277.204
|
| CAS号 |
93413-69-5
|
| 相关CAS号 |
Venlafaxine hydrochloride;99300-78-4;Venlafaxine-d6;1020720-02-8;Venlafaxine-d6-1;940297-06-3
|
| PubChem CID |
5656
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
397.6±27.0 °C at 760 mmHg
|
| 熔点 |
72-74°C
|
| 闪点 |
194.2±23.7 °C
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
| 折射率 |
1.544
|
| LogP |
2.91
|
| tPSA |
32.7
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
20
|
| 分子复杂度/Complexity |
279
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
PNVNVHUZROJLTJ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C17H27NO2/c1-18(2)13-16(17(19)11-5-4-6-12-17)14-7-9-15(20-3)10-8-14/h7-10,16,19H,4-6,11-13H2,1-3H3
|
| 化学名 |
1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl]cyclohexan-1-ol
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.6049 mL | 18.0245 mL | 36.0490 mL | |
| 5 mM | 0.7210 mL | 3.6049 mL | 7.2098 mL | |
| 10 mM | 0.3605 mL | 1.8025 mL | 3.6049 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Venlafaxine 25 mg Tablets Under Non-Fasting Conditions
CTID: NCT00834249
Phase: Phase 1 Status: Completed
Date: 2024-08-19