| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Viloxazine is rapidly absorbed following oral administration. The relative bioavailability of viloxazine extended-release relative to an immediate-release formulation was about 88%. Viloxazine Cmax and AUC increase proportionally over a dosage range from 100 mg to 600 mg once daily. The Cmax ranges between 540 and 1600 ng/mL. Following administration of a single 200 mg dose, the median Tmax was approximately five hours, with a range of three to nine hours. Steady-state was reached after two days of once-daily administration, and no accumulation was observed. A high-fat meal decreases Cmax and AUC by about 9% and 8%, respectively, and delays Tmax by two hours. Viloxazine is primarily excreted via renal elimination. After administration of radiolabeled viloxazine, 90% of the dose was recovered in urine within the first 24 hours post-dose. Less than 1% of the dose is excreted in the feces. About 12-15% of the total drug is eliminated as unchanged parent drug. The volume of distribution was 0.73 ± 0.28 L/kg following intravenous administration. The clearance rate was 124 ± 11 mL/hour/kg following intravenous administration. Metabolism / Metabolites Viloxazine undergoes CYP2D6-mediated 5-hydroxylation to form 5-hydroxyviloxazine. This metabolite can be glucuronidated by UGT1A9 and UGT2B15 to form 5-hydroxyviloxazine glucuronide, which is the major metabolite detected in plasma. Viloxazine can also be glucuronidated to form Viloxazine N-carbamoyl glucuronide. Biological Half-Life The mean (± SD) half-life of viloxazine was 7.02 (± 4.74) hours. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In four placebo-controlled trials of viloxazine in children with ADHD, minor serum aminotransferase elevations occurred in 5% to 10% of recipients but were more than 2 times the upper limit of normal in less than 1%. In the preregistration trials, there were no instances of clinically apparent liver injury or serum aminotransferase elevations with jaundice attributable to viloxazine. Since its approval as therapy for depression in Europe more than 30 years ago and as therapy of ADHD in the United States in 2021, there have been no publications describing clinically apparent liver injury due to viloxazine. Furthermore, summaries of the efficacy and safety of viloxazine do not mention hepatic adverse events. Nevertheless, long term clinical experience with viloxazine in children is limited, and other SNRIs (such as atomoxetine) have been linked to rare instances of clinically apparent liver injury. Likelihood score: E (unlikely cause of acute liver injury with jaundice). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation There is no published experience with viloxazine during breastfeeding. If viloxazine is required by the mother of an older infant, it is not a reason to discontinue breastfeeding, but until more data become available, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Viloxazine is 76-82% bound to human plasma proteins over the blood concentration range of 0.5 mcg/mL to 10 mcg/mL. |
| 其他信息 |
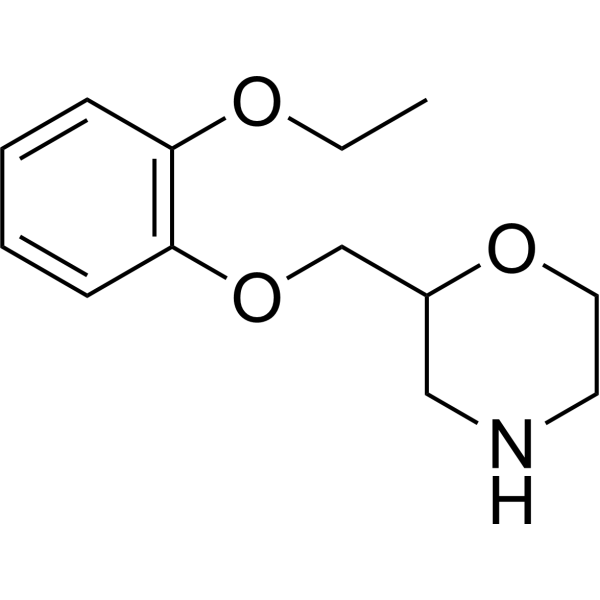
2-[(2-ethoxyphenoxy)methyl]morpholine is an aromatic ether.
Viloxazine is a selective norepinephrine reuptake inhibitor. For decades, an immediate-release formulation of viloxazine has been used in Europe as an antidepressant. It was first approved in the UK in 1974; however, the immediate-release formulation was discontinued due to business reasons unrelated to drug safety and efficacy. In the US, viloxazine was assigned an orphan drug designation in 1984 under the brand name CATATROL: while this product was intended to treat cataplexy and narcolepsy, the drug was never approved for these therapeutic indications. In April 2021, an extended-release formulation of viloxazine under the brand name QELBREE was approved by the FDA for the treatment of attention deficit hyperactivity disorder (ADHD). Viloxazine is a Norepinephrine Reuptake Inhibitor. The mechanism of action of viloxazine is as a Norepinephrine Uptake Inhibitor, and Cytochrome P450 1A2 Inhibitor, and Cytochrome P450 2D6 Inhibitor, and Cytochrome P450 3A4 Inhibitor, and Cytochrome P450 2B6 Inhibitor. Viloxazine is a selective norepinephrine reuptake inhibitor that is used in the therapy of attention deficit/hyperactivity disorder in children. Viloxazine has been associated with uncommon and mild serum enzyme elevations during therapy but not with occurrence of clinically apparent liver injury. A morpholine derivative used as an antidepressant. It is similar in action to IMIPRAMINE. See also: Viloxazine Hydrochloride (active moiety of). Drug Indication Viloxazine is a selective norepinephrine reuptake inhibitor indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in adults and pediatric patients 6 years and older. Mechanism of Action Attention Deficit Hyperactivity Disorder (ADHD) is a common neurodevelopmental disorder in children characterized by inattention and hyperactivity. In current literature, the pathophysiology of ADHD is understood to involve the imbalance of neurotransmitters, especially dopamine (DA) and norepinephrine (NE). The mechanism of action of viloxazine has not been fully elucidated; however, viloxazine is believed to work by modulating the monoaminergic neurotransmitter systems. Viloxazine is a selective and moderate norepinephrine reuptake inhibitor that binds to the norepinephrine transporter and inhibits the reuptake of norepinephrine. It thereby increases extracellular norepinephrine levels across several brain regions. Viloxazine potentiates serotonergic effects: it was shown to enhance neuronal sensitivity to serotonin and increase serotonin levels in the brain. _In vitro_, viloxazine is an antagonist at 5-HT2B receptors and an agonist 5-HT2C receptors. 5-HT2B receptors expressed on GABAergic interneurons are involved in tonic inhibitory control of serotonin neurons that innervate the medial prefrontal context; thus, antagonism of 5-HT2B receptors may result in disinhibition and enhanced serotonin release in the brain region. There is conflicting evidence in the literature that viloxazine increases dopamine levels in the brain via direct or indirect effects. For example, the norepinephrine transporter is also involved in the reuptake of dopamine in the prefrontal cortex and stimulation of 5-HT2C receptors facilitates DA release and enhances dopaminergic transmission in the brain. As dopamine dysregulation in the prefrontal cortex and amygdala is implicated in ADHD pathophysiology, the impact of viloxazine on dopamine levels may contribute to its mechanism of action. However, there is insufficient evidence to conclude this. Viloxazine has a negligible impact on dopamine in the nucleus accumbens and is not associated with an abuse risk. |
| 分子式 |
C13H19NO3
|
|---|---|
| 精确质量 |
237.136
|
| CAS号 |
46817-91-8
|
| 相关CAS号 |
Viloxazine hydrochloride;35604-67-2
|
| PubChem CID |
5666
|
| 外观&性状 |
Colorless to light yellow oil
|
| 密度 |
1.061 g/cm3
|
| 沸点 |
350.5ºC at 760 mmHg
|
| 熔点 |
185-186ºC
|
| 闪点 |
144.3ºC
|
| 折射率 |
1.499
|
| LogP |
1.781
|
| tPSA |
39.72
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
17
|
| 分子复杂度/Complexity |
213
|
| 定义原子立体中心数目 |
0
|
| SMILES |
C(OC1C=CC=CC=1OCC1CNCCO1)C
|
| InChi Key |
YWPHCCPCQOJSGZ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C13H19NO3/c1-2-15-12-5-3-4-6-13(12)17-10-11-9-14-7-8-16-11/h3-6,11,14H,2,7-10H2,1H3
|
| 化学名 |
2-[(2-ethoxyphenoxy)methyl]morpholine
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。