| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 靶点 |
ATP citrate lyase
|
|---|---|
| 体外研究 (In Vitro) |
藤黄提取物和HCA/羟基柠檬酸体外给药抑制HIF激活[2]
使用小鼠视网膜锥细胞系(661W)和人RPE细胞系(ARPE19)通过萤光素酶测定来评估HIF活性,因为光感受器和RPE细胞对AMD的发病机制有显著贡献,尽管AMD患者的iPS细胞衍生的类器官或分化细胞可以被认为是更好的体外系统。在缺氧条件下,HIFα脯氨酰羟化酶(PHD)的活性降低,导致HIFα稳定[12]。加入CoCl2以稳定PHD的抑制作用并激活HIF信号传导。Chetomin被用作HIF抑制剂的阳性对照。我们用藤黄提取物。表1列出了其成分,显示HCA占提取物的一半以上。与对照组相比,藤黄提取物和HCA在ARPE19细胞(图1A)和661W细胞(图1B)中显示出HIF抑制作用。[2] 藤黄果提取物和HCA/羟基柠檬酸下调Hif1a和下游基因[2] 我们研究了藤黄提取物和HCA如何影响Hif1a和下游基因的mRNA表达。在ARPE19细胞中,无论是否存在CoCl2,施用藤黄提取物都会显著下调Hif1a(图2A)。HIF的下游基因,如Vegfa、Bnip3和Pdk1,被CoCl2上调,被藤黄果提取物给药显著下调(图2B-D)。同样,在661W细胞中施用藤黄提取物后,Hif1a下调(图2E)。在661W细胞中,藤黄提取物的给药也下调了CoCl2诱导的Vegfa上调(图2F)。HIF的其他下游基因的表达与Vegfa一样有下调的趋势(图2G,H)。HCA还下调了ARPE19细胞(图3A-D)和661W细胞(图3E-H)中的Hif1a和下游基因。藤黄提取物和HCA均抑制了ARPE19细胞(图4A,B)和661W细胞(图4C,D)中CoCl2给药后HIF-1α蛋白表达的增加。 羟基柠檬酸(HCA)是一种经过验证的天然减肥剂,富含藤黄果。(科:Clusiaceae)。本研究使用HPLC和起始密码子靶向(SCoT)分子标记估算的HCA来评估35棵候选加树(CPT)的遗传变异性。还进行了表型和基因型性状之间的关联分析。选定的CPT显示平均HCA含量为29.11 mg/g,Gar 17最高(48.32 mg/g),其次是Gar 6(45.48 mg/g)。SCoT标记分析显示,30个引物中有19个引物共产生151条带,多态性带占66.89%。主坐标分析(PCoA)将CPT组织到散点图的四个象限中,而与HCA含量无关。基于邻域连接方法的树状图通过其自举值证明了其可重复性,并且它有三个聚类。结构分析揭示了所选个体中存在两个假设亚群的可能性。基于一般线性模型(GLM)的关联分析同意SCoT 5d等位基因与HCA含量的强关联,这也支持了Gar 6的前景。基于HCA和SCoT标记的分析有效地追踪了CPT之间的遗传变异和标记-性状关联。据我们所知,这是G.gummi-gutta的第一个发现[5]。 背景/目的:(-)-羟基柠檬酸(HCA)/Hydroxycitric acid已被证明可以抑制动物和人类的脂肪积累,但其潜在的生化机制尚不完全清楚,尤其是关于(-)-HCA是否调节能量代谢并因此影响脂肪沉积的信息很少。 方法:将肝细胞培养24小时,然后暴露于(-)-HCA(0、1、10、50µM)中,通过ELISA测定酶蛋白含量;RT-PCR检测脂质代谢基因mRNA水平。 结果:(-)-HCA显著减少了脂滴的数量和总面积。(-)-HCA处理后,ATP柠檬酸裂解酶、脂肪酸合酶和甾醇调节元件结合蛋白-1c mRNA水平显著降低,而过氧化物酶体增殖物激活受体αmRNA水平显著升高。(-)-HCA显著降低了细胞质中ATP柠檬酸裂解酶活性和乙酰辅酶A含量,但显著增加了葡萄糖消耗和线粒体耗氧率。(-)-HCA显著提高了葡萄糖激酶、磷酸果糖激酶-1、丙酮酸激酶、丙酮酸脱氢酶、柠檬酸合酶、乌头酶、琥珀酸脱氢酶、苹果酸脱氢酶、NADH脱氢酶和ATP合酶的活性/含量。 结论:(-)-HCA通过减少乙酰辅酶A的供应来减少脂滴的积聚,这主要是通过抑制ATP柠檬酸裂解酶和加速鸡肝细胞的能量代谢来实现的。这些结果从能量代谢的角度提出了(-)-HCA降低肉鸡脂肪的生化机制。[9] |
| 体内研究 (In Vivo) |
HCA/羟基柠檬酸治疗可以降低肾功能损害的标志物(血尿素氮和血清肌酐)。HCA治疗的小鼠草酸钙晶体沉积明显减少。草酸钙晶体诱导活性氧的产生,降低抗氧化防御酶的活性。HCA减轻了草酸钙结晶引起的氧化应激。HCA对草酸钙诱导的炎性细胞因子如MCP-1、IL-1β和IL-6具有抑制作用。此外,HCA减轻了草酸钙晶体引起的肾小管损伤和细胞凋亡。1.
给予羟基柠檬酸/HCA抑制了模型小鼠的CNV体积[2] 将悬浮在玉米油中的HCA以30mg/kg/天的剂量腹腔注射共两周,并在开始注射一周后用激光照射小鼠。与对照组相比,HCA给药组在照射第七天的CNV体积显著减少(图6A,B)。 给予羟基柠檬酸/HCA可抑制体内HIF-1α的表达[2] 将悬浮在玉米油中的HCA腹腔注射(30mg/kg/天)给小鼠共10天,并在给药的第七天用激光照射小鼠。在照射第三天的小鼠视网膜和脉络膜中,HIF-1α因激光照射而增加,因HCA的给药而受到抑制(图7A,B),尽管RPE/脉络膜组织的信号较弱。 共有135名受试者被随机分配到活性Hydroxycitric acid/羟基柠檬酸组(n=66)或安慰剂组(n=69);活性羟基柠檬酸组和安慰剂组分别有42例(64%)和42例(61%)完成了12周的治疗(P=0.74)。在12周的治疗期间,两组患者的体重均显著减轻(P<0.001);然而,组间体重减轻差异没有统计学意义(平均[SD],3.2[3.3]kg vs 4.1[3.9]kg;P=0.14)。治疗组之间估计的体脂质量损失百分比没有显著差异,受试者体重损失的脂肪比例不受治疗组的影响[4]。 目前的研究旨在探索(-)-羟基柠檬酸(HCA)对脂肪代谢的影响,并研究(-)-HCA的这种作用是否与调节鸡胚中葡萄糖-6-磷酸异构酶(GPI)的表达有关。我们构建了一个重组质粒(sh2-GPI)来抑制GPI的表达,然后用(-)-HCA处理胚胎。结果表明,(-)-HCA降低了脂滴积聚、甘油三酯含量和脂肪生成因子mRNA水平,增加了脂肪分解因子mRNA表达,而当用sh2 GPI预处理鸡胚时,(-”-HCA引起的这种作用被显著逆转。(-)-HCA增加了磷酸(p)-乙酰辅酶A羧化酶、烯酰辅酶A水合酶短链-1、肉碱棕榈酰基转移酶1A、p-AMP激活的蛋白激酶和过氧化物酶体增殖物激活的受体α蛋白的表达,当鸡胚用sh2 GPI预处理时,(-)-HCA的这种作用也消失了。这些数据表明,(-)-HCA通过激活AMPK途径减少脂肪沉积,而(-)HCA的减脂作用是由于鸡胚中GPI表达的增加。[8] (-)-羟基柠檬酸(HCA)是藤黄果提取物的主要活性成分,已被证明可以抑制动物和人类的体重增加和脂肪积累。然而,(-)-HCA的潜在机制尚未完全清楚。因此,本研究旨在探讨长期补充(-)-HCA对大鼠体重增加和氨基酸含量变化的影响。结果表明,(-)-HCA处理降低了大鼠的体重增加,提高了饲料转化率。(-)-HCA治疗组肝糖原、肌糖原、血清T4、T3、胰岛素和瘦素含量升高。(-)-HCA治疗组肝脏和肌肉中的蛋白质含量显著增加。氨基酸谱分析表明,(-)-HCA治疗组血清和肝脏中的大部分氨基酸含量,特别是芳香族氨基酸和支链氨基酸含量较高。然而,在(-)-HCA治疗组中,肌肉中的大部分氨基酸含量,特别是芳香族氨基酸和支链氨基酸,都降低了。这些结果表明,(-)-HCA治疗可以通过调节甲状腺激素水平来促进能量消耗,从而减少体重增加。此外,(-)-HCA治疗可以通过改变氨基酸的代谢方向来促进蛋白质合成[11]。 |
| 细胞实验 |
萤光素酶测定[2]
我们使用661W和ARPE19进行了萤光素酶测定,这两种都是转染的HIF萤光素酶报告基因构建体。这些构建体在HRE的控制下编码萤火虫荧光素酶基因,HRE如前所述结合HIF。作为内部对照,这些细胞与CMV肾荧光素酶构建体共转染。我们将0.8×104个细胞/孔/70μL的细胞接种在HTS Transwell®-96接收板上,白色,TC处理,无菌。接种后24小时,200μM CoCl2诱导HIF-αs。将藤黄提取物(藤黄提取物50%(表1))和羟基柠檬酸/HCA溶解在二甲亚砜中,并与CoCl2同时加入生长培养基中。考虑到材料的毒性,我们将溶解在DMSO中的每种化合物加入细胞培养基中,使其浓度为1mg/mL。给药后,细胞在37°C的5%CO2培养箱中孵育24小时。使用Dual luciferase®Reporter检测系统对萤光素酶表达进行定量。荧光强度由微孔板读数器读取。此外,使用100nM的chetomin作为HIF抑制剂的阳性对照,并使用不含CoCl2、藤黄果提取物和HCA的含DMSO培养基作为载体对照。 蛋白质印迹[2] 在体外实验中,我们向ARPE19细胞系中添加了200μM CoCl2、1 mg/mL藤黄提取物和羟基柠檬酸/HCA,同时考虑了材料的毒性。给药6小时后,将细胞收集在RIPA缓冲液中,并与蛋白酶抑制剂和MG132混合。然后,细胞被均质化。之后,我们对样品进行离心(14800 rpm,4°C,30分钟)并收集上清液。将蛋白质浓度调节至75μg/30μL。 羟基柠檬酸/HCA的估算[5] 根据Jayaprakasha和Sakariah(2000)的研究,从35个G.gummi gutta CPT的干果皮中提取并纯化HCA(Babu等人,2021;Vishnu等人,2022)。采用高效液相色谱法(HPLC)测定了35个甘米滴胶CPTs中HCA的含量。色谱系统由UFLC(日本京都岛津公司)、SPD-20A检测器、通信总线模块(CBM 20A)和Shim-pack GIST C18柱(5μm,4.6 ID×250 mm)组成。HCA的检测在210 nm处以0.1 AUFS的灵敏度进行。在等度条件下,使用0.6mM硫酸作为流动相,将流速设置为0.7ml/min。从羟基柠檬酸钾中纯化的HCA用作标准品。使用0.45μm PTFE注射器过滤器(I-131 m Axiva;印度Sonipat)过滤标准和样品,并使用20μl注射器将其注入系统。通过绘制不同HCA浓度(200、400、600、800和1000mg/l)与峰面积的关系来制备校准曲线。通过峰积分法估算选定CPT中HCA的浓度,并表示为mg/g样品。 |
| 动物实验 |
Male C57BL/6J mice were divided into a control group, glyoxylate(GOX) 100 mg/kg group, a GOX+HCA 100 mg/kg group, and a GOX+HCA/Hydroxycitric acid 200 mg/kg group. Blood samples and kidney samples were collected on the eighth day of the experiment. We used Pizzolato staining and a polarized light microscope to examine crystal formation and evaluated oxidative stress via the levels of malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px). Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) was used to detect the expression of monocyte chemotactic protein-1(MCP-1), nuclear factor-kappa B (NF κB), interleukin-1 β (IL-1 β) and interleukin-6 (IL-6) messenger RNA (mRNA). The expression of osteopontin (OPN) and a cluster of differentiation-44(CD44) were detected by immunohistochemistry and qRT-PCR. In addition, periodic acid Schiff (PAS) staining and TUNEL assay were used to evaluate renal tubular injury and apoptosis. [1]
Administration of Garcinia Extract and Hydroxycitric acid/HCA to Mice [2] An MF diet mixed with Garcinia extract at a concentration of 0.2% was administered to 4-week-old male mice for a total of 7 weeks while considering the toxicity of the material. The control group was administered an MF diet. The mice were irradiated with a laser 6 weeks after beginning administration. We injected 30 mg/kg/day HCA suspended in corn oil intraperitoneally to 6-week-old male mice 5 days/week for a total of 2 weeks. The control group was injected with corn oil. The laser was irradiated 1 week after the initial injection. Medications in vivo [3] Hydroxycitric acid tripotassium/K-HCA and citrate acid tripotassium/K-CA were tested as inhibitors to prevent the formation of stones. We randomly divided 600 flies into 6 groups (100 flies in each group) and fed them high-oxalate (0.05% NaOx) medium. Different concentrations (0.01%, 0.1%, and 1%) of K-HCA and K-CA were added in the medium of corresponding groups. Stone formation and life span were assessed in the same way as above. |
| 毒性/毒理 (Toxicokinetics/TK) |
In safety studies, acute oral toxicity, acute dermal toxicity, primary dermal irritation and primary eye irritation, were conducted in animals using various doses of HCA-SX. Results indicate that the LD50 of HCA-SX is greater than 5,000 mg/kg when administered once orally via gastric intubation to fasted male and female Albino rats. No gross toxicological findings were observed under the experimental conditions. Taken together, these in vivo toxicological studies demonstrate that HCA-SX is a safe, natural supplement under the conditions it was tested. Furthermore, HCA-SX can inhibit [3H]-5-HT uptake (and also increase 5-HT availability) in isolated rat brain cortical slices in a manner similar to that of SRRIs, and thus may prove beneficial in controlling appetite, as well as treatment of depression, insomnia, migraine headaches and other serotonin-deficient conditions.[10]
Adverse Events [1] No patient was removed from the study protocol for a treatment-related adverse event, and the number of reported adverse events was not significantly different between the placebo and treatment groups (eg, headache, 12 vs 9, respectively; upper respiratory tract symptoms, 13 vs 16, respectively; and gastrointestinal tract symptoms, 6 vs 13, respectively). |
| 参考文献 |
|
| 其他信息 |
Garcinia acid is a carbonyl compound.
See also: Garcinia gummi-gutta fruit (part of). Hydroxycitric acid is a carbonyl compound. Hydroxycitric acid has been reported in Garcinia cowa, Hibiscus sabdariffa, and Garcinia atroviridis with data available. See also: Hydroxycitric acid (annotation moved to). Background: Age-related macular degeneration (AMD) is the leading cause of blindness and can be classified into two types called atrophic AMD (dry AMD) and neovascular AMD (wet AMD). Dry AMD is characterized by cellular degeneration of the retinal pigment epithelium, choriocapillaris, and photoreceptors. Wet AMD is characterized by the invasion of abnormal vessels from the choroid. Although anti-vascular endothelial growth factor (VEGF) therapy has a potent therapeutic effect against the disease, there is a possibility of chorio-retinal atrophy and adverse systemic events due to long-term robust VEGF antagonism. We focused on hypoxia-inducible factor (HIF) regulation of VEGF transcription, and report the suppressive effects of HIF inhibition against ocular phenotypes in animal models. Many of the known HIF inhibitors are categorized as anti-cancer drugs, and their systemic side effects are cause for concern in clinical use. In this study, we explored food ingredients that have HIF inhibitory effects and verified their effects in an animal model of AMD. Methods: Food ingredients were screened using a luciferase assay. C57BL6/J mice were administered the Garcinia cambogia extract (Garcinia extract) and Hydroxycitric acid (HCA). Choroidal neovascularization (CNV) was induced by laser irradiation. Results: Garcinia extract and HCA showed inhibitory effects on HIF in the luciferase assay. The laser CNV model mice showed significant reduction of CNV volume by administering Garcinia extract and HCA. Conclusions: Garcinia extract and HCA showed therapeutic effects in a murine AMD model. Keywords: Garcinia cambogia; age-related macular degeneration; choroid; hydroxycitric acid; hypoxia-inducible factor; laser induced neovascularization; retina.[2] BACKGROUND Hydroxycitric acid is a potential lithontriptic agent for calcium oxalate (CaOx) stones in the kidneys. This study aimed to evaluate the safety and efficiency of hydroxycitric acid tripotassium (K-HCA) against CaOx crystal formation using Drosophila melanogaster hyperoxaluria models. MATERIAL AND METHODS Wild-type D. melanogaster were fed standard medium with ethylene glycol or sodium oxalate added to induce hyperoxaluria. Their Malpighian tubules were dissected and observed under a microscope every 3 days. Crystal deposit score of each Malpighian tubule were evaluated under a magnification of ×200. Using hyperoxaluria Drosophila models, we investigated the inhibitory efficiency of hydroxycitrate acid tripotassium and citric acid tripotassium (K-CA) against CaOx crystal formation. The survival rate of each group was also assessed. RESULTS When fed with 0.05% NaOx, the CaOx formation in Malpighian tubules increased significantly, without reduction of life span. Therefore, we selected 0.05% NaOx-induced hyperoxaluria models for the further investigations. After treatment, the stone scores showed that K-CA and K-HCA both significantly inhibit the formation of CaOx crystals in a dose-dependent manner, and with smaller dosage (0.01%), K-HCA was more efficient than K-CA. Moreover, after treatment of K-CA or K-HCA, the life span in different groups did not change, reflecting the safety to life. CONCLUSIONS The hyperoxaluria Drosophila models fed on 0.05% NaOx diet might be a useful tool to screen novel agents for the management of CaOx stones. K-HCA may be a promising agent for the prevention CaOx stones, with satisfying efficiency and acceptable safety. [3] Context: Hydroxycitric acid, the active ingredient in the herbal compound Garcinia cambogia, competitively inhibits the extramitochondrial enzyme adenosine triphosphate-citrate (pro-3S)-lyase. As a citrate cleavage enzyme that may play an essential role in de novo lipogenesis inhibition, G cambogia is claimed to lower body weight and reduce fat mass in humans. Objective: To evaluate the efficacy of G cambogia for body weight and fat mass loss in overweight human subjects. Design: Twelve-week randomized, double-blind, placebo-controlled trial. Setting: Outpatient weight control research unit. Participants: Overweight men and women subjects (mean body mass index [weight in kilograms divided by the square of height in meters], approximately 32 kg/m2). Intervention: Subjects were randomized to receive either active herbal compound (1500 mg of hydroxycitric acid per day) or placebo, and both groups were prescribed a high-fiber, low-energy diet. The treatment period was 12 weeks. Body weight was evaluated every other week and fat mass was measured at weeks 0 and 12. Main outcome measures: Body weight change and fat mass change. Results: A total of 135 subjects were randomized to either active hydroxycitric acid (n = 66) or placebo (n = 69); 42 (64%) in the active hydroxycitric acid group and 42 (61%) in the placebo group completed 12 weeks of treatment (P = .74). Patients in both groups lost a significant amount of weight during the 12-week treatment period (P<.001); however, between-group weight loss differences were not statistically significant (mean [SD], 3.2 [3.3] kg vs 4.1 [3.9] kg; P = .14). There were no significant differences in estimated percentage of body fat mass loss between treatment groups, and the fraction of subject weight loss as fat was not influenced by treatment group. Conclusions: Garcinia cambogia failed to produce significant weight loss and fat mass loss beyond that observed with placebo. [4] The moderate level of genetic diversity exhibited by the CPTs of G. gummi-gutta supports the precise amplification nature of SCoT markers and the availability of specific SCoT alleles within the species. Interrelationships shown by the individuals irrespective of Hydroxycitric acid/HCA content may be the result of gene flow through migrations. Gar1 was genetically distinct, obviously seen from the scatterplot and dendrogram. STRUCTURE analysis discovered the probability of two assumed subpopulations within the selected individuals. The highest concentration of HCA content was measured in Gar17. However, GLM analysis revealed the promising nature of accession Gar6 as evident by the strong association of its HCA content with SCoT 5d allele. The corresponding allele can significantly contribute to HCA content in the species, which could be taken into consideration while implementing breeding strategies in the species. The findings can be extended for marker-assisted selection and genetic improvement of G. gummi-gutta.[5] (−)-Hydroxycitric acid [(−)-HCA] is the principal acid of fruit rinds of Garcinia cambogia, Garcinia indica, and Garcinia atroviridis. (−)-HCA was shown to be a potent inhibitor of ATP citrate lyase (EC 4.1.3.8), which catalyzes the extramitochondrial cleavage of citrate to oxaloacetate and acetyl-CoA: citrate + ATP + CoA → acetyl-CoA + ADP + Pi + oxaloacetate. The inhibition of this reaction limits the availability of acetyl-CoA units required for fatty acid synthesis and lipogenesis during a lipogenic diet, that is, a diet high in carbohydrates. Extensive animal studies indicated that (−)-HCA suppresses the fatty acid synthesis, lipogenesis, food intake, and induced weight loss. In vitro studies revealed the inhibitions of fatty acid synthesis and lipogenesis from various precursors. However, a few clinical studies have shown controversial findings. This review explores the literature on a number of topics: the source of (−)-HCA; the discovery of (−)-HCA; the isolation, stereochemistry, properties, methods of estimation, and derivatives of (−)-HCA; and its biochemistry, which includes inhibition of the citrate cleavage enzyme, effects on fatty acid synthesis and lipogenesis, effects on ketogenesis, other biological effects, possible modes of action on the reduction of food intake, promotion of glycogenesis, gluconeogenesis, and lipid oxidation, (−)-HCA as weight-controlling agent, and some possible concerns about (−)-HCA, which provides a coherent presentation of scattered literature on (−)-HCA and its plausible mechanism of action and is provocative of further research.[6] Background: (-)-Hydroxycitric acid (HCA) is an active ingredient extracted from the rind of the Indian fruit Garcinia cambogia. It inhibits adenosine triphosphate citrate lyase and has been used in the treatment of obesity. Objective: The primary end point of this study was the effects of 12 weeks of G cambogia extract administration on visceral fat accumulation. The secondary end points were body indices (including height, body weight, body mass index [BMI], waist and hip circumference, and waist-hip ratio) and laboratory values (including total cholesterol, triacylglycerol, and free fatty acid). Methods: This study was performed according to a double-blind, randomized, placebo-controlled, parallel-group design. Subjects aged 20 to 65 years with a visceral fat area >90 cm(2) were enrolled. Subjects were randomly assigned to receive treatment for 12 weeks with G cambogia (containing 1000 mg of HCA per day) or placebo. At the end of the treatment period, both groups were administered placebo for 4 weeks to assess any rebound effect. Each subject underwent a computed tomography scan at the umbilical level at -2, 0, 12, and 16 weeks. Results: Forty-four subjects were randomized at baseline, and 39 completed the study (G cambogia group, n = 18; placebo group, n = 21). At 16 weeks, the G cambogia group had significantly reduced visceral, subcutaneous, and total fat areas compared with the placebo group (all indices P<0.001). No severe adverse effect was observed at any time in the test period. There were no significant differences in BMI or body weight at week 12, but there were slight numeric decreases in body weight and BMI in men. There were no signs of a rebound effect from week 12 to week 16. Conclusion: G cambogia reduced abdominal fat accumulation in subjects, regardless of sex, who had the visceral fat accumulation type of obesity. No rebound effect was observed. It is therefore expected that G cambogia may be useful for the prevention and reduction of accumulation of visceral fat.[7] |
| 分子式 |
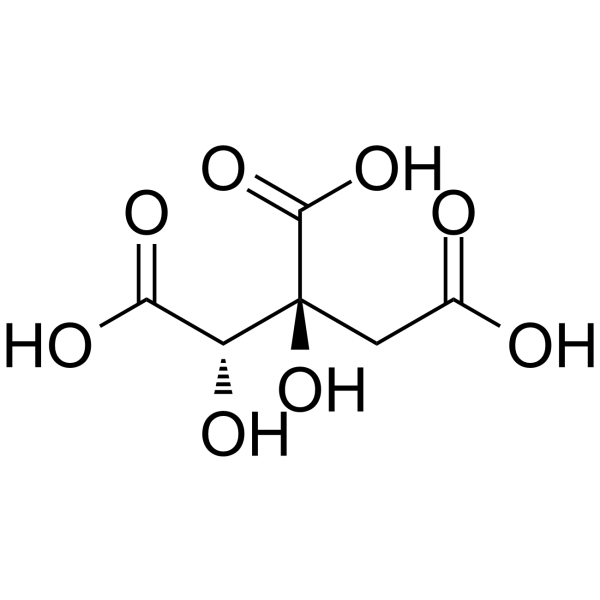
C6H8O8
|
|---|---|
| 分子量 |
208.12292
|
| 精确质量 |
529.885
|
| CAS号 |
27750-10-3
|
| 相关CAS号 |
Hydroxycitric acid;6205-14-7
|
| PubChem CID |
185620
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.947g/cm3
|
| 沸点 |
393.3ºC at 760mmHg
|
| 闪点 |
205.8ºC
|
| 蒸汽压 |
8.17E-08mmHg at 25°C
|
| 折射率 |
1.619
|
| LogP |
-2.6
|
| tPSA |
238.72
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
14
|
| 分子复杂度/Complexity |
271
|
| 定义原子立体中心数目 |
2
|
| SMILES |
C(C(=O)O)[C@]([C@@H](C(=O)O)O)(C(=O)O)O
|
| InChi Key |
ZMJBYMUCKBYSCP-CVYQJGLWSA-N
|
| InChi Code |
InChI=1S/C6H8O8/c7-2(8)1-6(14,5(12)13)3(9)4(10)11/h3,9,14H,1H2,(H,7,8)(H,10,11)(H,12,13)/t3-,6+/m1/s1
|
| 化学名 |
(1S,2S)-1,2-dihydroxypropane-1,2,3-tricarboxylic acid
|
| 别名 |
HYDROXYCITRATE, L-; 8W94T9026R; HYDROXYCITRIC ACID, (-)-; (1S,2S)-1,2-dihydroxy-1,2,3-propanetricarboxylic acid; Regulator; Haes cpd; Hydroxycitric acid ethylenediamine salt; ...; 27750-10-3;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~5 mg/mL (~24.02 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.8049 mL | 24.0246 mL | 48.0492 mL | |
| 5 mM | 0.9610 mL | 4.8049 mL | 9.6098 mL | |
| 10 mM | 0.4805 mL | 2.4025 mL | 4.8049 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。