| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Life-stage-dependent toxicity and dose-dependent toxicokinetics (TK) were evaluated in Sprague Dawley rats following dietary exposure to 2,4-dichlorophenoxyacetic acid (2,4-D). 2,4-D renal clearance is impacted by dose-dependent saturation of the renal organic anion transporter; thus, this study focused on identifying inflection points of onset of dietary nonlinear TK to inform dose selection decisions for toxicity studies. Male and female rats were fed 2,4-D-fortified diets at doses to 1600 ppm for 4-weeks premating, <2 weeks during mating, and to test day (TD) 71 to parental (P1) males and to P1 females through gestation/lactation to TD 96. F1 offspring were exposed via milk with continuing diet exposure until postnatal day (PND) 35. As assessed by plasma area under the curve for the time-course plasma concentration, nonlinear TK was observed > or = 1200 ppm (63 mg/kg/day) for P1 males and between 200 and 400 ppm (14-27 mg/kg/day) for P1 females. Dam milk and pup plasma levels were higher on lactation day (LD) 14 than LD 4. Relative to P1 adults, 2,4-D levels were higher in dams during late gestation/lactation and postweaning pups (PND 21-35) and coincided with elevated intake of diet/kg body weight. Using conventional maximum tolerated dose (MTD) criteria based on body weight changes for dose selection would have resulted in excessive top doses approximately 2-fold higher than those identified incorporating critical TK data. These data indicate that demonstration of nonlinear TK, if present at dose levels substantially above real-world human exposures, is a key dose selection consideration for improving the human relevance of toxicity studies compared with studies employing conventional MTD dose selection strategies. Distribution of 2,4-D occurs throughout the body, but there is no evidence that it is accumulated. Transformation in mammals appears to occur only to a slight extent & mainly involves the production of 2,4-D conjugates with sugars or amino acids. A single dose is excreted within a few days, mainly with the urine, & to a much lesser extent in the bile & feces. Pretreatment of rats with 2,4-D (250 mg/kg, sc) so occupied binding sites on plasma proteins that the distribution of (14)C-2,4-D admin iv 3.5 to 4.5 hr later was changed relative to controls, the concn being less in the plasma & kidney & greater in the liver, brain, spinal fluid, testis, lung, heart, & muscle. ... Human beings excrete 2,4-D mainly in the urine, & the blood plasma clearance times depend on the dose, individual characteristics, & the presence or absence of cmpds that may competitively inhibit 2,4-D excretion. For single oral doses of 2,4-D, the biological half-life in blood plasma is about one day, depending on the circumstances. However, forced alkaline diuresis may reduce this to as little as 3.7 hr. For more Absorption, Distribution and Excretion (Complete) data for 2,4-D (36 total), please visit the HSDB record page. Metabolism / Metabolites In ... studies with enzyme prepn from arthrobacter specie, 2,4-D was converted to 2,4-D phenol & glyoxylate. Condensation of the two glyoxylate molecules occurred with loss of CO2 from one carboxyl group. A cmpd chromatographically identical to alanine was observed. With ring-labeled 2,4-D, labeled succinate was produced. 2,4-D esters are hydrolyzed in animals. The phenoxy acids are excreted predominantly as such in the urine of rats after their oral admin, although minor portion is conjugated with amino acids glycine & taurine & with glucuronic acid. Soybean root callus cultures metabolized 2,4-D. Metabolites identified included 2,4-D-glutamic acid & 2,4-D-aspartic acid conjugates; other not identified 2,4-D amino acid conjugates; 2,5-dichloro-4-hydroxyphenoxyacetic acid (4-OH-2,5-D); and 5-OH-2,4-D ... in a comparison of 2,4-D metabolism by soybean callus, soybean plant and corn plants, no qualitative differences were observed. Hydroxy cmpd, mainly as glucosides, were identified as 5-OH-2,4-D, 4-OH-2,3-D, and 4-OH-2,5-D. Amino acid conjugates were identified as 2,4-D conjugates of aspartic acid, glutamic acid, alanine, valine, phenylalanine, tryptophan and leucine. There were some data that suggested the presence of amino acid conjugates of ring hydroxylated 2,4-D. /SRP: unspecified salt or ester of 2,4-D/ Male volunteers ingested single dose of 5 mg/kg. Excretion occurred mainly as 2,4-D (82.3%) with smaller amt as 2,4-D conjugate (12.8%). /SRP: unspecified salt or ester of 2,4-D/ For more Metabolism/Metabolites (Complete) data for 2,4-D (7 total), please visit the HSDB record page. Metabolism of 2,4-D is minimal in humans, with nearly all of it excreted unchanged as the parent compound. In particular, 2,4-D is rapidly excreted from the body, primarily in the urine. Much of the compound appears to be eliminated unchanged, although some 2,4-D is eliminated from the body as a conjugate. 2,4-D is metabolized to 2,4-dichlorophenol (2,4-DCP) by cytochrome P450 3A4 (CYP 3A4), the major form of monooxygenase enzyme in the human liver. Biological Half-Life Whole body: 220 hours (reduced to 4-7 hours by urinary alkalinization); mean plasma 1/2 life: 12 hours; [TDR, p. 510] ... The half-lives for urinary excretion were 3 hr in rats, 8 hr in calves, & hens, & about 12 hr in pigs. ... In rats orally or intravenously admin, 2,4-D is excreted primarily in the urine with a half-life of approx 2 hr. After six male volunteers each /orally/ ingested a subtoxic dose of 5 mg 2,4-D per kg, urine and blood samples were collected and monitored for 2,4-D levels. From pharmacokinetic analysis of the data, the half-life of plasma clearance was determined to be 33 hr. /SRP: Unspecified salt or ester of 2,4-D/ After ingestion of single 5 mg/kg oral dose ... /by 5 male human volunteers/, 2,4-D was eliminated from plasma in an apparent 1st order process with an avg /SRP: biological half-life/ of 11.7 hr. All subjects excreted 2,4-D in urine with avg /SRP: biological half-life/ of 17.7 hr, mainly as free 2,4-D (83.3%), with smaller amt excreted as 2,4-D conjugates (12.8%). /SRP: unspecified salt or ester of 2,4-D/ |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: 2,4-Dichlorophenoxyacetic acid (2,4-D) is an herbicide. It is a white powder with a slightly phenolic odor. It is soluble in water and the ester products of 2,4-D vary in solubility in water. It is used as a solid alkali salt concentrate or as a salt based water miscible solution or as an ester based emulsifiable concentrate; also used in mixtures with other herbicides. It is a component of Agent Orange, a military defoliant. It is used to control broad leaved weeds in cereals, grain crops, road sides and farm buildings and to increase latex output of old rubber trees. HUMAN EXPOSURE AND USE: 2,4-D may be absorbed from the gastrointestinal tract, by inhalation and to a lesser extent by the intact skin. Observations were made on 220 men exposed from 0.5 to 22 years of 2,4-D in a manufacturing plant. Medical evaluation revealed no difference when compared to a control group of 4600 men. In the exposed group, 10 men were karyotyped. There was no effect on the structural integrity or arrangement of the genetic material of the lymphocyte chromosomes. However, in an in vitro study, 2,4-D both in the presence and in the absence of the metabolic activator caused an increase in chromatid and chromosome breaks, number of micronuclei and number of nuclear buds. Presence of the S9 mix additionally elevated the number of chromatid breaks and micronuclei in treated lymphocytes. Signs and symptoms reported among workers at a plant manufacturing the amine salt and butyl ester included general weakness, rapid fatigue, frequent headache and vertigo. Cases of arterial hypotension were noted. There were possible indications of liver dysfunction which was noted in workers with long exposure to herbicides. In two groups of agricultural workers, 250 and 45 people respectively, excessive fatigue, epigastric pains, anorexia and occasional respiratory tract symptoms, and impaired taste sensitivity were reported. Reported cases of poisoning have been mainly the result of accidental or suicidal ingestion. Peripheral neuropathy has been reported along with contact dermatitis. ANIMAL STUDIES: It may be absorbed by the gastrointestinal tract, by inhalation or through intact skin. Studies in vivo on liver mitochondria have demonstrated that this herbicide uncouples oxidative phosphorylation at low concentrations. Young female rats were given various doses of 2,4-D orally by stomach tube five times a week for up to four weeks. At higher doses animals showed varying degrees of gastrointestinal irritation, slight cloudy swelling of the liver and depressed growth rate. High doses mortality was elevated due to severe gastrointestinal irritation. Accumulation of effect may occur in the form of liver or kidney damage but no clear cut biochemical lesion associated with prolonged exposure. Female rats were fed various levels of 2,4-D in their diet for up to two years. There was no significant difference in mortality between test and control groups. At autopsy of those animals who survived for the two year period there was no difference in body weight and hematological parameters were normal except in the final examination after 22 months revealed a possible tendency to macrocytosis, polychromasia and hypochromasia. Bile duct proliferation, slight hepatitis and nephritis occurred slightly more in test animals rather than controls. 2,4,-D is not considered a carcinogen. In a two year feeding study in rats there was a slight increase in tumor incidence in female rats, however the raw data did not show enough evidence to determine if 2,4-D is carcinogenic. In a number of developmental experiments in which rats, guinea pigs, hamsters and mice received high doses of 2,4-D there appeared to be an increased incidence of minor skeletal abnormalities. 2,4-D was also maternally toxic and embryolethal in rats, and it induced urogenital malformations in rat fetuses. The agent was also teratogenic and embryotoxic in mice. ECOTOXICITY STUDIES: Crayfish was exposed to three sublethal levels of 2,4-D for 96 hr and placed into a Y-maze system with a fish gelatin food source placed randomly in the right or left arm were impaired in their ability to forage effectively. These inabilities to locate and consume adequate amounts of food could result in lower body weights and decreased fitness in populations of crayfish exposed to 2,4-D in natural habitats. A mixture of 2,4-D and monosodium methanearsonate may compromise gill function, increasing the sensitivity of the crawfish to herbicide toxicity. 2,4-Dichlorophenoxyacetic acid is a strong oxidant and is known to cause lipid peroxidation and the generation of free radicals that can modify lipids and proteins. It is also known to inhibit glutathione S transferase which leads to a depletion of ATP, NADPH and glutathione (A3122, A3123). These actions can cause cell toxicity and apopotosis among metabolically active cells. Some of the endocrine effects of 2,4-D may be mediated by the 2,4-D mediated displacement of sex hormones from the sex hormone binding globulin or the 2,4-D mediated blocking or OAT6 transport proteins that are needed for the transport of functional organic ions and dicarboxylates (including estrone sulfate). Toxicity Data LC50 (rat) > 1,790 mg/m3 LD50: 1400 mg/kg (Dermal, Rabbit) (T14) LD50: 469 mg/kg (Oral, Guinea pig) LD50: 639 mg/kg (Oral, Rat) (L1982) LD50: 138 mg/kg (Oral, Mouse) (L1982) Interactions The toxic effects of a widely used herbicide (Dikamin D containing 72% 2,4-D-amine Na as active ingredient) applied alone or in combination with three heavy elements (copper sulphate, cadmium sulphate and lead acetate) modelling the heavy metal load of the environment were studied on chicken embryos with injection treatment. The treatment was done on day 0 of incubation. Solutions and emulsions of different concentrations were made from the test materials and injected in 0.1 mL volume into the air space of eggs. The macroscopical evaluations were done on day 19 of the incubation. Summarizing the findings, it can be established that the individual administration of the 72% 2,4-D containing herbicide formulation was less toxic compared to the control group than the simultaneous administration of the pesticide and heavy elements. As compared with each other the results from the combined administrations of the 72% 2,4-D containing herbicide formulation and heavy elements the simultaneous administration of cadmium and the herbicide caused the highest embryomortality while the incidence of developmental anomalies were the highest in the interaction study of the copper and the pesticide. /Dikamin D/ The effects of mixtures of parathion (PA;5 mg/kg), toxaphene (TOX; 50 mg/kg)and/or 2,4-dichlorophenoxyacetic acid (2,4-D; 50 mg/kg) on the hepatic mixed-function oxygenase (MFO) system were studied in ICR male mice (21-24 g) by oral intubation daily for 7 days. In general, TOX and TOX-containing mixtures were found to induce the metabolism of amidopyrine (21-52%), aniline (58-72%), phenacetin (239-307%), pentobarbital (104-148%) and benzo[a]pyrene (143-304%) in the 9000 g liver supernatants and to increase the hepatic cytochrome P-450 contents (57-80%). Furthermore, the TOX pretreatment was effective in enhancing the biotransformation of PA or paraoxon (PO) in the supernatants. This enhancement was not altered significantly by 5 mM EDTA. Although TOX increased the aliesterase activity in the serum and liver homogenates and supernatants by 31-158%, the activity of paraoxonase was not affected in these preparations. The TOX-induced increase in the metabolism of PA or PO was, at least in part, associated with the MFO system, and paraoxonase did not have significant involvement in the increase. These findings suggest that the toxicity of the PA + TOX mixture would be lower than that of PA, as TOX has the ability to increase the biotransformation of PA, as well as of PO, and the levels of aliesterase, thereby providing a pool of noncritical enzymes for the binding of PO. Because of these properties of TOX, it is anticipated that the toxicity of the PA + TOX + 2,4-D mixture also would be lower than that of PA. The effects of prenatal exposure to a 2,4-dichlorophenoxyacetic acid (24-D)/2,4,5-trichlorophenoxyacetic acid mixture on brain glutamate, gamma-aminobutyric acid (GABA), protein, DNA, and RNA were studied in rats. Pregnant Sprague Daley rats were orally administered 0, 50, or 125 mg/kg per day of a 1:1 mixture of 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid on gestational days six to 15. The mixture was known to contain 0.0125 ppm of 2,3,7,8-tetrachlorodienzo-p-dioxin. On postnatal days one, 15, or 22, brains of neonates were separated into cerebrum, cerebellum, neocortex and thalamus/hypothalamus, and assayed for glutamate, DNA, RNA, protein and gamma-aminobutyric acid. Regional brain concentrations of protein, DNA, and RNA were not affected by prenatal exposure to the mixture, except for a decrease in the protein/DNA ratio in the hypothlamus induced by the 50 and 125 mg/kg doses on postnatal day 22. Glutamate was significantly reduced in the cerebrum and cerebellum in 1 day old neonates exposed prenatally to 50 and 125 mg/kg 2,4-dichlorophenoxyacetic acid/2,4,5-trichlorophenoxyacetic acid, while levels were not significantly altered in offspring examined at 15 and 22 postnatal days. gamma-Aminobutyric acid was not significantly affected in any brain region at any time. Myotonia is characterized by prolonged contraction (delay in onset of relaxation) of skeletal muscle fibers with characteristic electromyographic findings. Calcium channel blocking drugs may be expected to reduce myotonia, should they promote the onset of relaxation in a contracted skeletal muscle. This study was aimed at evaluating the effect of diltiazem, a calcium channel blocking agent, on myotonia induced by 2,4-dichlorophenoxyacetic acid (2,4-D). In rat diaphragm, exposed to 2.5 mM 2,4-D in a tissue bath, myotonia was quantified by documenting the contraction time in response to direct stimulation with supramaximal electric stimuli. At a peak of myotonia, diltiazem was added to the tissue bath and the effect on evoked contraction studied over a period of 6 minutes. A concentration of 5 x 10(-5) M was found to be the most effective, causing a decrease in contraction time of more than 90% in 3 minutes. For more Interactions (Complete) data for 2,4-D (13 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse ingestion 521 mg/kg LD50 Rabbit skin 1400 mg/kg LD50 Rat Fischer-344 male oral 443 mg/kg (95% confidence limits 270-1103 mg/kg) (2,4-D acid in corn oil). LD50 for 3 week old chicks (M, F) by oral administration of undiluted 2,4-D/2,4,5-T (1:1) was 4000 mg/kg (2700-5900 mg/kg). Acid equivalent was not specified. /From table/ For more Non-Human Toxicity Values (Complete) data for 2,4-D (8 total), please visit the HSDB record page. |
| 其他信息 |
2,4-Dichlorophenoxyacetic acid (2,4-D) does not occur naturally in the environment. 2,4-D is the active ingredient in many products used in the United States and throughout the world as an herbicide to kill weeds on land and in the water. There are nine forms of 2,4-D that can be used as an herbicide and it is typically sold as a powder or in a liquid form.
2,4-d sodium salt appears as a clear brown to black liquid with a characteristic phenoxy odor. Primary hazard is threat to the environment. Immediate steps should be taken to limit spread to the environment. Easily penetrates the soil to contaminate groundwater and nearby waterways. 2,4-dichlorophenoxyacetic acid is an odorless white to tan solid. Sinks in water. (USCG, 1999) 2,4-D is a chlorophenoxyacetic acid that is phenoxyacetic acid in which the ring hydrogens at postions 2 and 4 are substituted by chlorines. It has a role as a synthetic auxin, a defoliant, an agrochemical, an EC 1.1.1.25 (shikimate dehydrogenase) inhibitor, an environmental contaminant and a phenoxy herbicide. It is a chlorophenoxyacetic acid and a dichlorobenzene. It is a conjugate acid of a (2,4-dichlorophenoxy)acetate. 2,4-Dichlorophenoxyacetic acid has been reported in Guanomyces polythrix, Nicotiana tabacum, and Phoma herbarum with data available. 2,4-Dichlorophenoxyacetic acid (2,4-D) is a common systemic herbicide used in the control of broadleaf weeds. It is the most widely used herbicide in the world, and the third most commonly used in North America. 2,4-D is also an important synthetic auxin, often used in laboratories for plant research and as a supplement in plant cell culture media such as MS medium. (S685). 2,4-D can be formulated as emulsifiable concentrates, granules, soluble concentrate and solids, water-dispersible granules, and wettable powders. 2,4-D is used alone, but is commonly formulated with dicamba, mecoprop, mecoprop-p, MCPA, and clopyralid. 2,4-D was one of the ingredients in Agent Orange, the herbicide widely used during the Vietnam War. Though 2,4-D composed 50% of Agent Orange, the health effects of Agent Orange are related to dioxin contaminants generated during the production of Agent Orange – not 2,4-D itself. On August 8, 2007, the U.S. Environmental Protection Agency issued a ruling that stated that existing data do not support a link between human cancer and 2,4-D exposure. An herbicide with irritant effects on the eye and the gastrointestinal system. Mechanism of Action The widely used hormonal herbicide, 2,4-dichlorophenoxyacetic acid, blocks meiotic maturation in vitro and is thus a potential environmental endocrine disruptor with early reproductive effects. To test whether maturation inhibition was dependent on protein kinase A, an endogenous maturation inhibitor, oocytes were microinjected with PKI, a specific PKA inhibitor, and exposed to 2,4-D. Oocytes failed to mature, suggesting that 2,4-D is not dependent on PKA activity and likely acts on a downstream target, such as Mos. De novo synthesis of Mos, which is triggered by mRNA poly(A) elongation, was examined. Oocytes were microinjected with radiolabelled in vitro transcripts of Mos RNA and exposed to progesterone and 2,4-D. RNA analysis showed progesterone-induced polyadenylation as expected but none with 2,4-D. 2,4-D-activated MAPK was determined to be cytoplasmic in localization studies but poorly induced Rsk2 phosphorylation and activation. In addition to inhibition of the G2/M transition, 2,4-D caused abrupt reduction of H1 kinase activity in MII phase oocytes. Attempts to rescue maturation in oocytes transiently exposed to 2,4-D failed, suggesting that 2,4-D induces irreversible dysfunction of the meiotic signaling mechanism. Chlorophenoxy herbicides are chemical analogues of auxin, a plant growth hormone, and produce uncontrolled and lethal growth in target plants. Male Wistar rats were treated daily by gavage with ... 2,4-D (100-200 mg/kg body wt) ... induced proliferation of hepatic peroxisomes, decr serum lipid levels, incr hepatic carnitine acetyltransferase, and catalase. ... Data suggest ... compounds cause hypolipidemia, ... by preferentially incr lipid utilization in the liver. ... 2,4-dichlorophenoxyacetic acid (2,4-D) is a hormonal herbicide widely used in the world because of its efficacy in the control of broadleaf and woody plants. In this study we have demonstrated in vivo covalent binding of the phenoxyherbicide 2,4-D to a single protein of 52 kD (from rat liver mitochondrial preparation) detected through immunoblotting studies with the specific antiserum for 2,4-D. The direct involvement of 2,4-D in the formation of the adduct has also been demonstrated in vitro, using liver mitochondrial preparations exposed to (14)C-UL-2,4-D. Radiolabeled protein separated by SDS-PAGE and afterwards electroeluted showed a single labeled protein of 52 kD. When mitochondria exposed to radiolabeled xenobiotic were devoid of their outer membrane, the specific activity observed suggest that protein involved in covalent interaction belongs to the inner mitochondrial membrane. We propose that covalent binding of the phenoxyherbicide 2,4-D to a very specific single protein of 52 kD observed in vitro and in vivo may be related to known alterations of the mitochondrial function. |
| 分子式 |
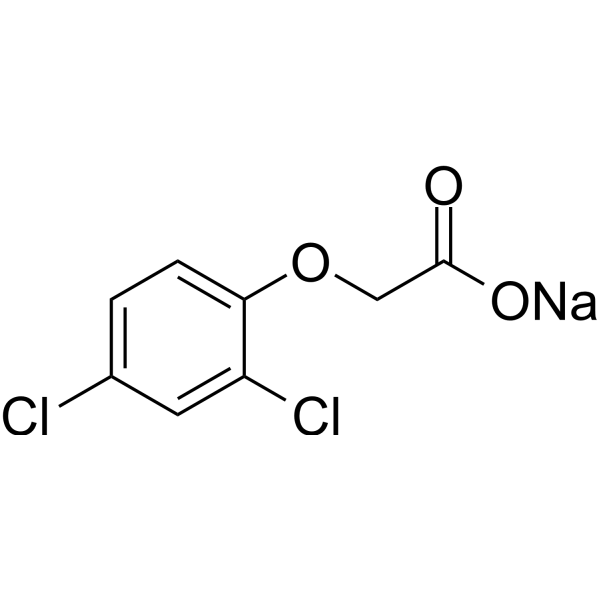
C₈H₅CL₂NAO₃
|
|---|---|
| 分子量 |
243.02
|
| 精确质量 |
241.951
|
| CAS号 |
2702-72-9
|
| 相关CAS号 |
2,4-D;94-75-7
|
| PubChem CID |
1486
|
| 外观&性状 |
White to yellow crystalline powder /SRP: yellow color is phenolic impurities/
Colorless powder White to yellow, crystalline ... powder Crystals from benzene |
| 沸点 |
345.6ºC at 760 mmHg
|
| 熔点 |
215°C
|
| 闪点 |
162.8ºC
|
| 蒸汽压 |
2.31E-05mmHg at 25°C
|
| LogP |
1.122
|
| tPSA |
49.36
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
13
|
| 分子复杂度/Complexity |
186
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1C([H])=C(C([H])=C([H])C=1OC([H])([H])C(=O)O[H])Cl
|
| InChi Key |
RFOHRSIAXQACDB-UHFFFAOYSA-M
|
| InChi Code |
InChI=1S/C8H6Cl2O3.Na/c9-5-1-2-7(6(10)3-5)13-4-8(11)12;/h1-3H,4H2,(H,11,12);/q;+1/p-1
|
| 化学名 |
sodium;2-(2,4-dichlorophenoxy)acetate
|
| 别名 |
2,4D sodium salt; 2,4 D sodium salt
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.1149 mL | 20.5744 mL | 41.1489 mL | |
| 5 mM | 0.8230 mL | 4.1149 mL | 8.2298 mL | |
| 10 mM | 0.4115 mL | 2.0574 mL | 4.1149 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。