| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
IC50: 30 nM (FLAP)[3] IC50: 3 nM (Leukotriene biosynthesis in intact leukocytes) and 1.1 μM (Leukotriene biosynthesis in human whole blood)[2] PPARα[1]
|
|---|---|
| 体外研究 (In Vitro) |
在小鼠原代角质形成细胞培养物中,MK-886 钠盐(0.5-2 μM;15 小时)处理可降低 keratin-1 表达[1]。使用瞬时转染技术,10 μM MK-886 钠盐可将猴肾成纤维细胞 CV-1 细胞、小鼠角化细胞 308 细胞和人肺腺癌 A549 细胞中 PPARα 的 Wy-14643 活化降低约 80%。在稳定转染系统中,MK-886 钠盐还可减少脂肪酸对 PPARα 的激活 [1]。尽管 Jurkat 细胞中表达所有 PPAR 同工型,但不同的 PPARα 和 PPARγ 激动剂无法阻止 MK-886 钠盐诱导的细胞凋亡[1]。
|
| 体内研究 (In Vivo) |
用 MK-886 钠盐(L 663536;5 mg/kg;口服)治疗的雄性 Sprague-Dawley 大鼠对先前接受过美西麦角治疗的近交系大鼠显示出对抗原诱导的呼吸困难的强烈抑制作用[2]。使用大鼠胸膜炎(ED50,0.2 mg/kg 口服)、炎症爪(ED50,0.8 mg/kg)和抗原激发后大鼠胆汁中白三烯排泄的模型,MK-886 钠盐 (L 663536) 抑制白三烯的产生体内[2]。
|
| 酶活实验 |
PPAR ligand binding assay: MK886与PPARs的结合采用共激活剂依赖受体配体测定(CARLA)。从Walter Wahli获得了一个包含PPARα配体结合域的结构体,该结构体与谷胱甘肽s -转移酶(GST)在框架中克隆。GST-PPAR配体结合域融合蛋白在大肠杆菌BL2 DE3 (pLysS)中表达。将含有融合蛋白的细菌微球重悬于10ml裂解缓冲液[含有1% (v}v) Triton X-100和0.5 mM PMSF的PBS]中,反复冻融裂解。4500 g离心除去DNA和不溶性物质。融合蛋白在4℃下用GSH-Sepharose beads纯化,在裂解缓冲液中洗涤3次,并在20 mM Tris}HCl (pH 8.0)、100 mM NaCl、1 mM EDTA、0.5% Nonidet P-40和1 mM添加1% (w}v)干牛奶的二硫苏糖醇(DTT)中平衡。每次反应使用的蛋白质量为1-3µg。将微球与不同浓度的MK886和$& s放射性标记的类固醇受体共激活因子-1 (SRC-1)一起孵育,SRC-1是由pSG5质粒构建的,该质粒可以在体外表达SRC-1(来自Walter Wahli),使用偶联转录翻译兔网状细胞裂解液系统。标记通过在[$&S]蛋氨酸存在下孵育1小时来实现,并通过离心回收珠子。然后洗涤,用SDS}PAGE分析与SRC-1的相互作用。gst配体结合区域融合的考马斯亮蓝染色允许不同反应之间的标准化。放射自显影显示SRC-1蛋白复合物。使用扫描图像和UN-SCAN-IT软件确定相对频带密度[1]。
|
| 细胞实验 |
蛋白质印迹分析[1]
细胞类型: 原代角质形成细胞 测试浓度: 0.5 µM、1 µM 或 2 µM 孵育时间:15小时 实验结果:keratin-1表达减少。 |
| 动物实验 |
Animal/Disease Models: Male SD (SD (Sprague-Dawley)) rat (300-400 g), antigen-induced dyspnea [1]
Doses: 5 mg/kg Route of Administration: Oral Experimental Results:Inhibition of antigen-induced dyspnea. |
| 参考文献 |
[1]. Inhibition of peroxisome-proliferator-activated receptor (PPAR)alpha by MK886. Biochem J. 2001 Jun 15;356(Pt 3):899-906.
[2]. L-663,536 (MK-886) (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2 - dimethylpropanoic acid), a novel, orally active leukotriene biosynthesis inhibitor. Can J Physiol Pharmacol. 1989 May;67(5):456-64. [3]. Mancini JA, et al. 5-Lipoxygenase-activating protein is the target of a novel hybrid of two classes of leukotriene biosynthesis inhibitors. Mol Pharmacol. 1992 Feb;41(2):267-72. |
| 其他信息 |
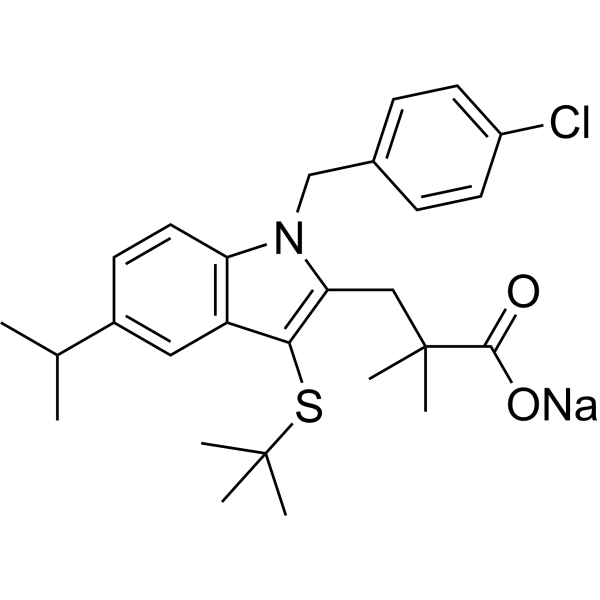
3-[3-(tert-butylsulfanyl)-1-(4-chlorobenzyl)-5-(propan-2-yl)-1H-indol-2-yl]-2,2-dimethylpropanoic acid is a member of the class of indoles that is 1H-indole substituted by a isopropyl group at position 5, a tert-butylsulfanediyl group at position 3, a 4-chlorobenzyl group at position 1 and a 2-carboxy-2-methylpropyl group at position 2. It acts as an inhibitor of arachidonate 5-lipoxygenase. It has a role as an EC 1.13.11.34 (arachidonate 5-lipoxygenase) inhibitor, an antineoplastic agent and a leukotriene antagonist. It is an aryl sulfide, a member of indoles, a monocarboxylic acid and a member of monochlorobenzenes.
MK-886 is an experimental inhibitor of leukotriene synthesis. Although MK886 was originally identified as an inhibitor of 5-lipoxygenase activating protein (FLAP), recent data demonstrate that this activity does not underlie its ability to induce apoptosis [Datta, Biswal and Kehrer (1999) Biochem. J. 340, 371--375]. Since FLAP is a fatty-acid binding protein, it is conceivable that MK886 may affect other such proteins. A family of nuclear receptors that are activated by fatty acids and their metabolites, the peroxisome-proliferator-activated receptors (PPARs), have been implicated in apoptosis and may represent a target for MK886. The ability of MK886 to inhibit PPAR-alpha, -beta and -gamma activity was assessed using reporter assay systems (peroxisome-proliferator response element--luciferase). Using a transient transfection system in monkey kidney fibroblast CV-1 cells, mouse keratinocyte 308 cells and human lung adenocarcinoma A549 cells, 10--20 microM MK886 inhibited Wy14,643 activation of PPAR alpha by approximately 80%. Similar inhibition of PPAR alpha by MK886 was observed with a stable transfection reporter system in CV-1 cells. Only minimal inhibitory effects were seen on PPAR beta and PPAR gamma. MK886 inhibited PPAR alpha by a non-competitive mechanism as shown by its effects on the binding of arachidonic acid to PPAR alpha protein, and a dose-response study using a transient transfection reporter assay in COS-1 cells. An assay assessing PPAR ligand-receptor interactions showed that MK886 prevents the conformational change necessary for active-complex formation. The expression of keratin-1, a protein encoded by a PPAR alpha-responsive gene, was reduced by MK886 in a culture of mouse primary keratinocytes, suggesting that PPAR inhibition has functional consequences in normal cells. Although Jurkat cells express all PPAR isoforms, various PPAR alpha and PPAR gamma agonists were unable to prevent MK886-induced apoptosis. This is consistent with MK886 functioning as a non-competitive inhibitor of PPAR alpha, but may also indicate that PPAR alpha is not directly involved in MK886-induced apoptosis. Although numerous PPAR activators have been identified, the results show that MK886 can inhibit PPAR alpha, making it the first compound identified to have such an effect.[1] L-663,536 (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2, 2-dimethylpropanoic acid) is a potent inhibitor of leukotriene (LT) biosynthesis in intact human polymorphonuclear leukocytes (PMN) (IC50, 2.5 nM). Similarly, L-663,536 inhibited A23187-induced LTB4 formation by rat peripheral blood and elicited PMN. At concentrations where inhibition of leukotriene biosynthesis occurred in human whole blood (1.1 microM), no effect was seen on cyclooxygenase or 12-lipoxygenase, an effect also observed in washed human platelets. The compound had no effect on rat or porcine 5-lipoxygenase indicating that L-663,536 is not a direct 5-lipoxygenase inhibitor. When administered in vivo L-663,536 was a potent inhibitor of antigen-induced dyspnea in inbred rats pretreated with methysergide (ED50, 0.036 mg/kg p.o.) and of Ascaris-induced bronchoconstriction in squirrel monkeys (1 mg/kg p.o.). The compound inhibited leukotriene biosynthesis in vivo in a rat pleurisy model (ED50, 0.2 mg/kg p.o.), an inflamed rat paw model (ED50, 0.8 mg/kg), a model of leukotriene excretion in rat bile following antigen provocation, and a model in the guinea-pig ear where leukotriene synthesis was induced by topical challenge with ionophore A23187 (ED50, 2.5 mg/kg p.o. and 0.6 micrograms topically). The results indicate that L-663,536 is a potent inhibitor of leukotriene biosynthesis both in vitro and in vivo indicating that the compound is suitable for studying the role of leukotrienes in a variety of pathological situations.[2] An 18-kDa leukocyte membrane protein, termed 5-lipoxygenase-activating protein (FLAP), has recently been shown to be the target of two structurally distinct classes of leukotriene biosynthesis inhibitors. These classes of inhibitors are based on indole and quinoline structures and are represented by MK-886 and L-674,573, respectively. A novel class of hybrid structure based on the indole and quinoline classes of inhibitors, termed quindoles, has recently been developed. These compounds, exemplified by L-689,037, are potent inhibitors of leukotriene biosynthesis, both in vitro and in vivo. In the present study, we have developed and characterized a potent radioiodinated photoaffinity analogue of L-689,037, termed [125I]L-691,678. This compound was used in immunoprecipitation studies with FLAP antisera to show that the quindole series of leukotriene biosynthesis inhibitors interact directly with FLAP. In addition, we show that MK-886, L-674,573, and L-689,037 specifically compete, in a concentration-dependent manner, with both [125I]L-691,678 and [125I]L-669,083, a photoaffinity analogue of MK-886, for binding to FLAP. These results suggest that these three classes of leukotriene biosynthesis inhibitors share a common binding site on FLAP, providing further evidence that FLAP represents a suitable target for structurally diverse classes of leukotriene biosynthesis inhibitors.[3] |
| 分子式 |
C27H33CLNNAO2S
|
|---|---|
| 分子量 |
494.06
|
| 精确质量 |
493.181
|
| 元素分析 |
C, 65.64; H, 6.73; Cl, 7.18; N, 2.84; Na, 4.65; O, 6.48; S, 6.49
|
| CAS号 |
118427-55-7
|
| 相关CAS号 |
MK-886;118414-82-7
|
| PubChem CID |
4519262
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| LogP |
6.675
|
| tPSA |
70.36
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
645
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC(C)C1=CC2=C(C=C1)N(CC3=CC=C(C=C3)Cl)C(=C2SC(C)(C)C)CC(C)(C)C(=O)[O-].[Na+]
|
| InChi Key |
CBNCIYNCWVGEKJ-UHFFFAOYSA-M
|
| InChi Code |
InChI=1S/C27H34ClNO2S.Na/c1-17(2)19-10-13-22-21(14-19)24(32-26(3,4)5)23(15-27(6,7)25(30)31)29(22)16-18-8-11-20(28)12-9-18;/h8-14,17H,15-16H2,1-7H3,(H,30,31);/q;+1/p-1
|
| 化学名 |
sodium;3-[3-tert-butylsulfanyl-1-[(4-chlorophenyl)methyl]-5-propan-2-ylindol-2-yl]-2,2-dimethylpropanoate
|
| 别名 |
MK-886 sodium salt; 118427-55-7; MK886 sodium; MK-886 (sodium salt); PNR27O326B; sodium;3-[3-tert-butylsulfanyl-1-[(4-chlorophenyl)methyl]-5-propan-2-ylindol-2-yl]-2,2-dimethylpropanoate; CHEMBL416657; MK 886 sodium;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0240 mL | 10.1202 mL | 20.2405 mL | |
| 5 mM | 0.4048 mL | 2.0240 mL | 4.0481 mL | |
| 10 mM | 0.2024 mL | 1.0120 mL | 2.0240 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。