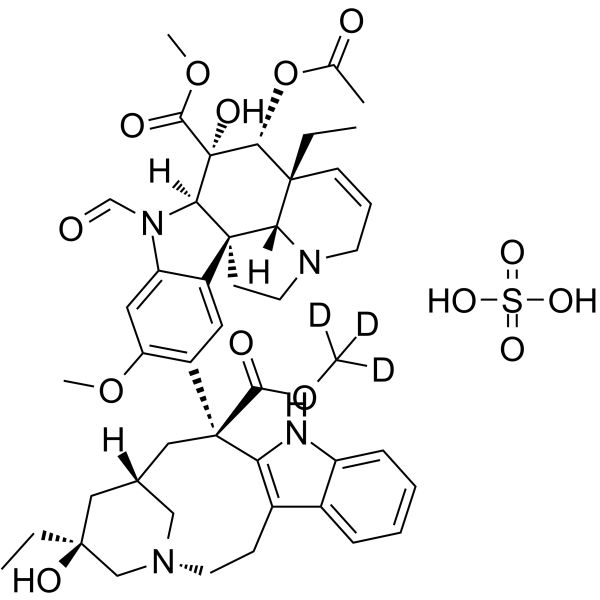
长春新碱-d3-酯(硫酸盐)是硫酸长春新碱的氘代形式。硫酸长春新碱是一种抗肿瘤长春花生物碱,可以抑制有丝分裂纺锤体中微管的形成,导致分裂细胞在中期停滞。其微管结合的 Ki 为 85 nM。
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
A Study to Compare Standard Therapy to Treat Hodgkin Lymphoma to the Use of Two Drugs, Brentuximab Vedotin and Nivolumab
CTID: NCT05675410
Phase: Phase 3 Status: Recruiting
Date: 2024-12-02
Nivolumab in Combination With Chemo-Immunotherapy for the Treatment of Newly Diagnosed Primary Mediastinal B-Cell Lymphoma
CTID: NCT04759586
Phase: Phase 3 Status: Recruiting
Date: 2024-12-02
A Phase 2 Study of Ruxolitinib With Chemotherapy in Children With Acute Lymphoblastic Leukemia
CTID: NCT02723994
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-11-29
Treatment of Acute Lymphoblastic Leukemia in Children
CTID: NCT00400946
Phase: Phase 3 Status: Completed
Date: 2024-11-27
Ibrutinib, Rituximab, Venetoclax, and Combination Chemotherapy in Treating Patients with Newly Diagnosed Mantle Cell Lymphoma
CTID: NCT03710772
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-11-27
View More
Testing CC-486 (Oral Azacitidine) Plus the Standard Drug Therapy in Patients 75 Years or Older With Newly Diagnosed Diffuse Large B Cell Lymphoma
CTID: NCT04799275
Phase: Phase 2/Phase 3 Status: Recruiting
Date: 2024-11-27
Testing the Use of Steroids and Tyrosine Kinase Inhibitors With Blinatumomab or Chemotherapy for Newly Diagnosed BCR-ABL-Positive Acute Lymphoblastic Leukemia in Adults
CTID: NCT04530565
Phase: Phase 3 Status: Recruiting
Date: 2024-11-25
A Study of the Drugs Selumetinib Versus Carboplatin/Vincristine in Patients With Neurofibromatosis and Low-Grade Glioma
CTID: NCT03871257
Phase: Phase 3 Status: Recruiting
Date: 2024-11-22
Blinatumomab, Inotuzumab Ozogamicin, and Combination Chemotherapy as Frontline Therapy in Treating Patients With B Acute Lymphoblastic Leukemia
CTID: NCT02877303
Phase: Phase 2 Status: Recruiting
Date: 2024-11-20
A Study of the Drugs Selumetinib vs. Carboplatin and Vincristine in Patients With Low-Grade Glioma
CTID: NCT04166409
Phase: Phase 3 Status: Recruiting
Date: 2024-11-19
Combination Chemotherapy With or Without Donor Stem Cell Transplant in Treating Patients With Acute Lymphoblastic Leukemia
CTID: NCT00792948
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-11-13
Brentuximab Vedotin and Combination Chemotherapy in Treating Children and Young Adults With Stage IIB, Stage IIIB, IVA, or IVB Hodgkin Lymphoma
CTID: NCT02166463
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-11-13
Combination Chemotherapy With or Without Bortezomib in Treating Younger Patients With Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or Stage II-IV T-Cell Lymphoblastic Lymphoma
CTID: NCT02112916
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-11-13
Combination Chemotherapy in Treating Young Patients With Newly Diagnosed High-Risk B Acute Lymphoblastic Leukemia and Ph-Like TKI Sensitive Mutations
CTID: NCT02883049
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-11-13
Blinatumomab in Treating Younger Patients With Relapsed B-cell Acute Lymphoblastic Leukemia
CTID: NCT02101853
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-11-13
Rituximab and Combination Chemotherapy With or Without Lenalidomide in Treating Patients With Newly Diagnosed Stage II-IV Diffuse Large B Cell Lymphoma
CTID: NCT01856192
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-11-13
Vorinostat, Rituximab, and Combination Chemotherapy in Treating Patients With Newly Diagnosed Stage II, Stage III, or Stage IV Diffuse Large B-Cell Lymphoma
CTID: NCT00972478
Phase: Phase 1/Phase 2 Status: Active, not recruiting
Date: 2024-11-13
Obinutuzumab With or Without Umbralisib, Lenalidomide, or Combination Chemotherapy in Treating Patients With Relapsed or Refractory Grade I-IIIa Follicular Lymphoma
CTID: NCT03269669
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-11-12
Testing the Addition of a New Anti-cancer Drug, Venetoclax, to Usual Chemotherapy for High Grade B-cell Lymphomas
CTID: NCT03984448
Phase: Phase 2/Phase 3 Status: Active, not recruiting
Date: 2024-11-12
Ibrutinib, Rituximab, Etoposide, Prednisone, Vincristine Sulfate, Cyclophosphamide, and Doxorubicin Hydrochloride in Treating Patients With HIV-Positive Stage II-IV Diffuse Large B-Cell Lymphomas
CTID: NCT03220022
Phase: Phase 1 Status: Recruiting
Date: 2024-11-12
A Study to Compare Early Use of Vinorelbine and Maintenance Therapy for Patients With High Risk Rhabdomyosarcoma
CTID: NCT04994132
Phase: Phase 3 Status: Recruiting
Date: 2024-11-08
Venetoclax and Vincristine in Treating Patients With Relapsed or Refractory T-cell or B-cell Acute Lymphoblastic Leukemia
CTID: NCT03504644
Phase: Phase 1/Phase 2 Status: Active, not recruiting
Date: 2024-11-06
Comparison of Radiation Therapy Regimens in Combination With Chemotherapy in Treating Young Patients With Newly Diagnosed Standard-Risk Medulloblastoma
CTID: NCT00085735
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-11-05
Nivolumab With DA-REPOCH Chemotherapy Regimen in Treating Patients With Aggressive B-Cell Non-Hodgkin's Lymphoma
CTID: NCT03749018
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-11-04
Irinotecan and Carboplatin as Upfront Window Therapy in Treating Patients With Newly Diagnosed Intermediate-Risk or High-Risk Rhabdomyosarcoma
CTID: NCT00077285
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-11-04
Zanubrutinib in Combination with R-PolaCHP (ZaR-PolaCHP) for Newly Diagnosed Diffuse Large B-Cell Lymphoma
CTID: NCT04850495
Phase: Phase 1 Status: Recruiting
Date: 2024-10-31
A Study to Compare Blinatumomab Alone to Blinatumomab With Nivolumab in Patients Diagnosed With First Relapse B-Cell Acute Lymphoblastic Leukemia (B-ALL)
CTID: NCT04546399
Phase: Phase 2 Status: Suspended
Date: 2024-10-30
Testing the Addition of 131I-MIBG or Lorlatinib to Intensive Therapy in People With High-Risk Neuroblastoma (NBL)
CTID: NCT03126916
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-10-26
Venetoclax and a Pediatric-Inspired Regimen for the Treatment of Newly Diagnosed B Cell Acute Lymphoblastic Leukemia
CTID: NCT05157971
Phase: Phase 1 Status: Recruiting
Date: 2024-10-26
Inotuzumab Ozogamicin and Frontline Chemotherapy in Treating Young Adults With Newly Diagnosed B Acute Lymphoblastic Leukemia
CTID: NCT03150693
Phase: Phase 3 Status: Suspended
Date: 2024-10-26
Reduced Craniospinal Radiation Therapy and Chemotherapy in Treating Younger Patients With Newly Diagnosed WNT-Driven Medulloblastoma
CTID: NCT02724579
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-10-26
Blinatumomab and Combination Chemotherapy or Dasatinib, Prednisone, and Blinatumomab in Treating Older Patients With Acute Lymphoblastic Leukemia
CTID: NCT02143414
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-10-23
Inotuzumab Ozogamicin and Post-Induction Chemotherapy in Treating Patients With High-Risk B-ALL, Mixed Phenotype Acute Leukemia, and B-LLy
CTID: NCT03959085
Phase: Phase 3 Status: Recruiting
Date: 2024-10-22
Combination Chemotherapy, Autologous Stem Cell Transplant, and/or Radiation Therapy in Treating Young Patients With Extraocular Retinoblastoma
CTID: NCT00554788
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-10-22
A Study of Revumenib in Combination With Chemotherapy for Patients Diagnosed With Relapsed or Refractory Leukemia
CTID: NCT05761171
Phase: Phase 2 Status: Recruiting
Date: 2024-10-22
A Study to Investigate Blinatumomab in Combination With Chemotherapy in Patients With Newly Diagnosed B-Lymphoblastic Leukemia
CTID: NCT03914625
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-10-21
Risk-Based Therapy in Treating Younger Patients With Newly Diagnosed Liver Cancer
CTID: NCT00980460
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-10-21
Combination Chemotherapy in Treating Young Patients With Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or T-cell Lymphoblastic Lymphoma
CTID: NCT00408005
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-10-21
Imatinib Mesylate and Combination Chemotherapy in Treating Patients With Newly Diagnosed Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia
CTID: NCT03007147
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-10-18
A Study of Daratumumab and Dose-Adjusted EPOCH in Plasmablastic Lymphoma
CTID: NCT04139304
PhaseEarly Phase 1 Status: Active, not recruiting
Date: 2024-10-16
Cisplatin and Combination Chemotherapy in Treating Children and Young Adults With Hepatoblastoma or Liver Cancer After Surgery
CTID: NCT03533582
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-10-15
Combination Chemotherapy With or Without Temsirolimus in Treating Patients With Intermediate Risk Rhabdomyosarcoma
CTID: NCT02567435
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-10-01
Combination Chemotherapy Followed By Peripheral Stem Cell Transplant in Treating Young Patients With Newly Diagnosed Supratentorial Primitive Neuroectodermal Tumors or High-Risk Medulloblastoma
CTID: NCT00336024
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-10-01
Combination Chemotherapy With or Without Rituximab in Treating Patients With Newly Diagnosed Non-Hodgkin's Lymphoma
CTID: NCT00004112
Phase: Phase 3 Status: Completed
Date: 2024-09-23
Testing the Addition of an Anti-cancer Drug, Lenalidomide, to the Usual Combination Chemotherapy Treatment ('EPOCH') for Adult T-Cell Leukemia-Lymphoma (ATL)
CTID: NCT04301076
Phase: Phase 1 Status: Recruiting
Date: 2024-09-20
Chemotherapy and Radiation Therapy in Treating Young Patients With Newly Diagnosed, Previously Untreated, High-Risk Medulloblastoma/PNET
CTID: NCT00392327
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-09-19
Combination Chemotherapy in Treating Patients With Non-Metastatic Extracranial Ewing Sarcoma
CTID: NCT01231906
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-09-19
Parsaclisib Plus the Standard Drug Therapy in Patients with Newly Diagnosed, High Risk Diffuse Large B-cell Lymphoma
CTID: NCT04323956
Phase: Phase 1 Status: Active, not recruiting
Date: 2024-09-19
Treating Young Patients With Newly Diagnosed, Low Stage, Lymphocyte Predominant Hodgkin Disease
CTID: NCT00107198
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-09-19
Tailored Prednisone Reduction in Preventing Hyperglycemia in Participants With B-Cell Non-Hodgkin Lymphoma Receiving Combination Chemotherapy Treatment
CTID: NCT03505762
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-09-19
Combination Chemotherapy and Nelarabine in Treating Patients with T-cell Acute Lymphoblastic Leukemia or Lymphoblastic Lymphoma
CTID: NCT00501826
Phase: Phase 2 Status: Recruiting
Date: 2024-09-19
Combination Chemotherapy With or Without Ganitumab in Treating Patients With Newly Diagnosed Metastatic Ewing Sarcoma
CTID: NCT02306161
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-09-05
Lenalidomide, Rituximab, and Combination Chemotherapy in Treating Patients With Newly Diagnosed Stage II, Stage III, or Stage IV Diffuse Large Cell or Follicular B-Cell Lymphoma
CTID: NCT00670358
Phase: Phase 1/Phase 2 Status: Active, not recruiting
Date: 2024-08-29
Azacitidine and Combination Chemotherapy in Treating Infants With Acute Lymphoblastic Leukemia and KMT2A Gene Rearrangement
CTID: NCT02828358
Phase: Phase 2 Status: Active, not recruiting
Date: 2024-08-09
Combination Chemotherapy and Inotuzumab Ozogamicin in Treating Patients With B Acute Lymphoblastic Leukemia
CTID: NCT03488225
Phase: Phase 2 Status: Terminated
Date: 2024-07-16
Busulfan, Melphalan, and Stem Cell Transplant After Chemotherapy in Treating Patients With Newly Diagnosed High-Risk Neuroblastoma
CTID: NCT01798004
Phase: Phase 1 Status: Completed
Date: 2024-07-15
Combination Chemotherapy With
Phase 2 Study of Pembrolizumab and Chemotherapy in Patients With Newly Diagnosed Classical Hodgkin Lymphoma (KEYNOTE-C11)
CTID: null
Phase: Phase 2 Status: Trial now transitioned, Ongoing
Date: 2021-07-30
A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study of Acalabrutinib in Combination with Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (R-CHOP) in Subjects ≤75 Years with Previously Untreated Non-Germinal Center Diffuse Large B-Cell Lymphoma.
CTID: null
Phase: Phase 3 Status: Trial now transitioned, Ongoing
Date: 2020-11-30
Brain Re-Irradiation Or Chemotherapy: a phase II randomised trial of re-irradiation and chemotherapy in patients with recurrent glioblastoma
CTID: null
Phase: Phase 2 Status: GB - no longer in EU/EEA
Date: 2020-07-06
An Open-label, Uncontrolled, Multicenter Phase II Trial of MK-3475 (Pembrolizumab) in Children and Young Adults with Newly Diagnosed Classical Hodgkin Lymphoma with Inadequate (Slow Early) Response to Frontline Chemotherapy (KEYNOTE 667).
CTID: null
Phase: Phase 2 Status: Trial now transitioned, GB - no longer in EU/EEA
Date: 2019-06-14
Open-label, Single-arm Trial to Evaluate Antitumor Activity, Safety, and Pharmacokinetics of Isatuximab Used in Combination With Chemotherapy in Pediatric
CTID: null
Phase: Phase 2 Status: Ongoing, Completed
Date: 2019-03-04
STELLAR: A phase II, randomiSed study of CHOP-R in combination with acalabruTinib comparEd to CHOP-R in patients with newLy diagnosed Richter’s Syndrome (RS) and a pLAtfoRm for initial investigations into activity of novel treatments in relapsed/refractory and newly diagnosed RS.
CTID: null
Phase: Phase 2 Status: GB - no longer in EU/EEA
Date: 2019-01-31
A PHASE III, MULTICENTER, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED TRIAL COMPARING THE EFFICACY AND SAFETY OF POLATUZUMAB VEDOTIN IN COMBINATION WITH RITUXIMAB AND CHP (R-CHP) VERSUS RITUXIMAB AND CHOP (R-CHOP) IN PREVIOUSLY UNTREATED PATIENTS WITH
CTID: null
Phase: Phase 3 Status: Completed, Trial now transitioned, GB - no longer in EU/EEA, Ongoing
Date: 2018-08-31
A multi-center, open-label, non-randomized, phase I dose escalation study of regorafenib (BAY 73-4506) in pediatric subjects with solid malignant tumors that are recurrent or refractory to standard therapy.
CTID: null
Phase: Phase 1 Status: Ongoing, Completed
Date: 2018-01-15
Paediatric Hepatic International Tumour Trial
CTID: null
Phase: Phase 3 Status: Ongoing, GB - no longer in EU/EEA, Completed
Date: 2017-04-28
Risk-stratified sequential Treatment with Ibrutinib and Rituximab (IR) and IR-CHOP for De-novo post-transplant Lymphoproliferative disorder (PTLD)
CTID: null
Phase: Phase 2 Status: GB - no longer in EU/EEA
Date: 2016-09-23
Phase III Randomized Clinical Trial of Lurbinectedin (PM01183)/Doxorubicin (DOX) versus Cyclophosphamide (CTX), Doxorubicin (DOX) and Vincristine (VCR) (CAV) or Topotecan as Treatment in Patients with Small-Cell Lung Cancer (SCLC) Who Failed One Prior Platinum-containing Line (ATLANTIS Trial)
CTID: null
Phase: Phase 3 Status: Completed
Date: 2016-06-28
A phase 2 study of brentuximab vedotin in combination with standard of care treatment (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone [RCHOP]) or RCHP (rituximab, cyclophosphamide, doxorubicin, and prednisone) as front-line therapy in patients with diffuse large B-cell lymphoma (DLBCL)
CTID: null
Phase: Phase 2 Status: Completed
Date: 2016-04-05
A Phase 1b-2, Open-Label, Dose Escalation and Expansion Study Evaluating the Safety and Efficacy of Entospletinib (ENTO [GS-9973]) combined with Vincristine (VCR) in Adult Subjects with Relapsed or Refractory B-cell Non-Hodgkin Lymphoma (NHL)
CTID: null
Phase: Phase 2 Status: Completed
Date: 2016-02-03
Randomised, open label study of rituximab/ibrutinib vs rituximab/chemotherapy in older patients with untreated mantle cell lymphoma
CTID: null
Phase: Phase 2, Phase 3 Status: Trial now transitioned, GB - no longer in EU/EEA
Date: 2015-09-30
Vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia: comparing one-hour infusions with short-term infusions (the VINCA-study)
CTID: null
Phase: Phase 4 Status: Completed
Date: 2014-08-25
ROMIDEPSIN IN COMBINATION WITH CHOEP AS FIRST LINE TREATMENT BEFORE HEMATOPOIETIC STEM CELL TRANSPLANTATION IN YOUNG PATIENTS WITH NODAL PERIPHERAL T-CELL LYMPHOMAS: A PHASE I-II STUDY.
CTID: null
Phase: Phase 1, Phase 2 Status: Ongoing
Date: 2014-05-27
Classification of Newly Diagnosed Acute Lymphoblastic Leukemia (ALL)
CTID: null
Phase: Phase 3 Status: Prematurely Ended
Date: 2014-03-07
Treatment of Patients with Newly Diagnosed Standard Risk B-Lymphoblastic Leukemia (B-ALL) or Localized B-lineage Lymphoblastic Lymphoma (B-LLy)
CTID: null
Phase: Phase 3 Status: Prematurely Ended
Date: 2014-01-14
A PHASE III MULTICENTER, RANDOMIZED STUDY COMPARING CONSOLIDATION WITH (90)YTTRIUM-LABELED IBRITUMOMAB TIUXETAN (ZEVALIN®) RADIOIMMUNOTHERAPY VS AUTOLOGOUS STEM CELL TRANSPLANTATION (ASCT) IN PATIENTS WITH RELAPSED FOLLICULAR LYMPHOMA (FL) AGED 18-65 YEARS
CTID: null
Phase: Phase 3 Status: Ongoing
Date: 2013-10-08
Pilot study to investigate the early prediction of toxicity following induction chemotherapy in Ewing’s sarcoma by blood-borne biomarkers and correlation with age-dependent pharmacokinetic variation
CTID: null
Phase: Phase 4 Status: GB - no longer in EU/EEA
Date: 2013-10-07
A randomized, double-blind, placebo-controlled, phase 3 study of brentuximab vedotin and CHP (A+CHP) versus CHOP in the frontline treatment of patients with CD30-positive mature T-cell lymphomas
CTID: null
Phase: Phase 3 Status: Completed
Date: 2013-02-18
International Randomised Controlled Trial for the Treatment of Newly Diagnosed Ewing's Sarcoma Family of Tumours
CTID: null
Phase: Phase 3 Status: Ongoing, GB - no longer in EU/EEA
Date: 2013-02-01
R-CHOP-14 or R-CHOP-21 & consolidation PET–oriented radiotherapy (RT) in diffuse large B cell lymphoma (DLBCL) patients with low risk profile according to age-adjusted IPI (0 with bulky or 1)
CTID: null
Phase: Phase 2 Status: Completed
Date: 2012-09-27
Reinduction protocol for patients with high-risk neuroblastoma in first relapse
CTID: null
Phase: Phase 2 Status: Ongoing
Date: 2012-09-20
A multicenter, phase III, randomized study to evaluate the efficacy of a response-adapted strategy to define maintenance after standard chemoimmunotherapy in patients with advanced-stage Follicular Lymphoma
CTID: null
Phase: Phase 3 Status: Ongoing
Date: 2012-08-21
DIAGNOSTIC AND THERAPEUTIC STUDY FOR NEWLY DIAGNOSED RETINOBLASTOMA PATIENTS RTB AIEOP 012
CTID: null
Phase: Phase 2 Status: Prematurely Ended
Date: 2012-02-14
United Kingdom National Randomised Trial for Children and Young Adults with Acute Lymphoblastic Leukaemia and Lymphoma 2011
CTID: null
Phase: Phase 3 Status: Ongoing, GB - no longer in EU/EEA
Date: 2011-12-02
Improvement of outcome and reduction of toxicity in elderly patients with CD20+ aggressive B-cell lymphoma by an optimised schedule of the monoclonal antibody rituximab, substitution of conventional by liposomal vincristine and FDG-PET based reduction of therapy in combination with vitamin D substitution
CTID: null
Phase: Phase 3 Status: Completed
Date: 2011-10-10
Phase II trial on safety and activity of intensive short-term chemoimmunotherapy in HIV-positive patients with Burkitt's lymphoma.
CTID: null
Phase: Phase 2 Status: Prematurely Ended
Date: 2011-10-03
A randomised evaluation of molecular guided therapy for diffuse large B-cell lymphoma with Bortezomib
CTID: null
Phase: Phase 3 Status: GB - no longer in EU/EEA
Date: 2010-12-20
ACNS0331
CTID: null
Phase: Phase 3 Status: Ongoing
Date: 2010-04-14
Traitement adjuvant dans les rétinoblastomes unilatéraux étendus énucléés d’emblée.
CTID: null
Phase: Phase 2 Status: Trial now transitioned
Date: 2009-12-15
A Randomized, Open-Label, Multicenter, Phase 2 Study of the Combination of
CTID: null
Phase: Phase 2 Status: Completed
Date: 2009-12-04
Phase I/II Study combining humanised anti-CD20 (veltuzumab), anti-CD22 (epratuzumab) and both monoclonal antibodies with chemotherapy in adults with recurrent B precursor acute lymphoblastic leukaemia (ALL)- MARALL
CTID: null
Phase: Phase 2 Status: Prematurely Ended
Date: 2009-08-10
Phase ll study evaluating the toxicity and efficacy of a modified German Paediatric Hodgkin's Lymphoma protocol (HD95) in young adults (aged 18-30 years) with Hodgkin's Lymphoma
CTID: null
Phase: Phase 2 Status: Completed
Date: 2008-07-11
A Randomised, Open-Label, Multicentre Phase 3 Study of the Combination of
CTID: null
Phase: Phase 3 Status: Completed
Date: 2008-04-29
An Open-Label, Randomized, Phase 3 Study of Inotuzumab Ozogamicin (CMC-544) Administered in Combination With Rituximab Compared to a Defined Investigator’s Choice Therapy in Subjects With Relapsed or Refractory, CD22- Positive, Follicular B-Cell Non Hodgkin’s Lymphoma
CTID: null
Phase: Phase 3 Status: Prematurely Ended, Completed
Date: 2008-04-25
A Phase 2 Study to Evaluate the Safety and Efficacy of Weekly Doses of Marqibo® (vincristine sulfate liposomes injection) in Adult Patients with Philadelphia Chromosome-negative Acute Lymphoblastic Leukemia (ALL) in Second Relapse or Adult Patients with Philadelphia Chromosome-negative ALL Who Failed Two Treatment Lines of Anti-leukemia Chemotherapy
CTID: null
Phase: Phase 2 Status: Prematurely Ended, Completed
Date: 2007-11-21
Feasibility study of R-CHOP plus bevacizumab in patients with diffuse large B cell lymphoma (DLBCL)
CTID: null
Phase: Phase 2 Status: Prematurely Ended
Date: 2007-03-02
Use of mieloablative doses of zevalin in aggressive lymphomas of the elderly. Prospective randomized study Z-HDS1,2 vs R-CHOP
CTID: null
Phase: Phase 3 Status: Prematurely Ended
Date: 2006-09-20
Etude multicentrique randomisée de phase III en ouvert comparant l'association Velcade Dexamethasone à la chimiothérapie de type VAD pour le traitement des patients porteurs de myélome multiple de novo jusqu'à l'âge de 65 ans
CTID: null
Phase: Phase 3 Status: Completed
Date: 2006-07-04
Cooperative multicentre study for children and adolescents with low grade glioma
CTID: null
Phase: Phase 3 Status: Ongoing, Completed
Date: 2006-05-03
Phase III multicentric IIL study, three randomized arms (R-CVP vs R-CHOP vs R-FM),for treatment of patients with stage II-IV follicular lymphoma
CTID: null
Phase: Phase 3 Status: Completed
Date: 2006-01-30
PROTOCOL OF DIAGNOSIS AND THERAPY FOR RETINOBLASTOMA - AIEOP RB 05
CTID: null
Phase: Phase 3 Status: Prematurely Ended
Date: 2006-01-01
A phase II GISL study of R-HyperCVAD in the treatment of patients with Mantle cell Lymphoma
CTID: null
Phase: Phase 2 Status: Completed
Date: 2005-09-06
Pilotstudie zur Therapieoptimierungsstudie für den
CTID: null
Phase: Phase 4 Status: Completed
Date: 2005-05-19
MULTI-CENTRE, RANDOMISED, PHASE III TRIAL COMPARING HIGH DOSE SEQUENTIAL CHEMOTHERAPY hds WITH RITUXIMAB AND AUTOLOGOUS PERIPHERAL BLOOD PROGENITUR ALL TRANSPLANTION VERSUS 2- WEEKLY CHOP WITH RITUXIMAB AS FRONT LINE THERAPY OF HIGH RISK PATIENT WITH DIFFUSE LARGE B- CELL NON HODGKIN LYMPHOMA
CTID: null
Phase: Phase 3 Status: Completed
Date: 2005-04-12
A phase II study for the treatment of patients with splenic marginal lymphoma with the combination of Cyclophosfamide, Vincristine, Liposomal Doxorubicin, Predinisone and Rituximab
CTID: null
Phase: Phase 2 Status: Completed
Date: 2005-02-24
Evaluation of the intensification of post-remissional therapy in the treatment of high-risks adult Acute Lymphoblastic Leukemia and monitoring of the minimal residual disease
CTID: null
Phase: Phase 3 Status: Completed
Date: 2004-11-08
IntReALL HR 2010
CTID: null
Phase: Phase 2 Status: Trial now transitioned, Ongoing, Prematurely Ended
Date: