| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
阿莫地喹(10–20 μM;4 小时)治疗以剂量依赖性方式抑制脂多糖 (PLS) 引起的促炎细胞因子(IL-1β、白细胞介素 6、TNF-α 和 iNOS)的表达 [1 ]。 TH+ 神经元数量和多巴胺摄取分析表明,阿莫地喹(5 μM;24 小时)可有效防止神经毒性 (6-OHDA) 诱导的原发性多巴胺细胞死亡。此外,在大鼠 PC12 细胞中观察到阿莫地喹。莫迪喹的神经保护作用[1]
|
|---|---|
| 体内研究 (In Vivo) |
阿莫地喹(40 mg/kg;腹膜内;每日;持续 3 天)治疗可减少雄性 ICR 小鼠的星形胶质细胞和小胶质细胞/巨噬细胞的血肿周围激活。除了改善小鼠的运动障碍外,阿莫地喹还可以降低 ICH 诱导的 IL-1β、CCL2 和 CXCL2 mRNA 表达 [2]。
|
| 细胞实验 |
RT-PCR[1]
细胞类型:原代小胶质细胞 测试浓度: 10 µM、15 µM、20 µM 孵育时间:4小时 实验结果:抑制LPS诱导的促炎细胞因子(IL-1β、白细胞介素6、TNF-α和iNOS) )以剂量依赖性方式。 |
| 动物实验 |
Animal/Disease Models: Male ICR mice (8-10 weeks old) induced intracerebral hemorrhage (ICH) [2]
Doses: 40 mg/kg Route of Administration: intraperitoneal (ip) injection; daily; lasted for 3 days Experimental Results: Microglia around the hematoma / diminished activation of macrophages and astrocytes. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Rapidly absorbed following oral administration. Amodiaquine hydrochloride is readily absorbed from the gastrointestinal tract. It is rapidly converted in the liver to the active metabolite desethylamodiaquine, which contributes nearly all of the antimalarial effect (10). There are insufficient data on the terminal plasma elimination half-life of desethylamodiaquine. Both amodiaquine and desethylamodiaquine have been detected in the urine several months after administration. After oral administration amodiaquine hydrochloride is rapidly absorbed... After oral administration of amodiaquine (600 mg) to 7 healthy adult males ... The peak concentration of amodiaquine was 32 +/- 3 ng/mL at 0.5 +/- 0.03 hr. The peak concentrations of amodiaquine in whole blood and packed cells were 60 +/- 10 and 42 +/- 6 ng/mL respectively, reached at 0.5+/- 0.1hr in both. Thereafter the concentration of amodiaquine declined rapidly, and was detectable for no more than 8 hr. Mean peak plasma concentration of the metabolite (desethylamodiaquine) was 181 +/- 26 ng/mL. Times to peak for whole blood and packed cells were 2.2 +/- 0.5 and 3.6 +/- 1.1 hr respectively For more Absorption, Distribution and Excretion (Complete) data for AMODIAQUINE (10 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic biotransformation to desethylamodiaquine (the principal biologically active metabolite) is the predominant route of amodiaquine clearance with such a considerable first pass effect that very little orally administered amodiaquine escapes untransformed into the systemic circulation. ... Amodiaquine hydrochloride ... undergoes rapid and extensive metabolism to desethylamodiaquine which concentrates in blood cells. It is likely that desethylamodiaquine, not amodiaquine, is responsible for most of the observed antimalarial activity, and that the toxic effects of amodiaquine after oral administration may in part be due to desethylamodiaquine. When amodiaquine is given orally relatively little of the parent compound is present in the blood. Hepatic biotransformation to desethylamodiaquine (the principal biologically active metabolite) is the predominant route of amodiaquine clearance with such a considerable first pass effect that very little orally administered amodiaquine escapes untransformed into the systemic circulation. The hepatic metabolism of the antimalarial drug amodiaquine was investigated in order to gain further insight into the postulated metabolic causation of the hepatotoxicity, which restricts the use of the drug. After intraportal administration (54 mumol/kg) to the anaesthetized rat, the drug was excreted in bile (23 +/- 3% dose over 5 h; mean +/- SD, n = 6) primarily as thioether conjugates. After intraportal administration, 20% of the dose was excreted into urine over 24 h as parent compound and products of N-dealkylation and oxidative deamination. Desethylamodiaquine accumulated in liver, but was not a substrate for bioactivation as measured by biliary elimination of a glutathione adduct. Prior administration of ketoconazole, an inhibitor of P450, reduced biliary excretion by 50% and effected a corresponding decrease in the amount of drug irreversibly bound to liver proteins. This indicated a role for P450 in the bioactivation of amodiaquine to a reactive metabolite that conjugates with glutathione and protein. De-ethylation and irreversible binding were observed in vitro using male rat liver microsomes, and were again inhibited by ketoconazole. However, no such binding was observed with human (six individuals) hepatic microsomes despite extensive turnover of amodiaquine to desethylamodiaquine. Amodiaquine quinoneimine underwent rapid reduction in the presence of either human or rat liver microsomes. Therefore in vitro studies may underestimate the bioactivation of amodiaquine in vivo. These data indicate that the extent of protein adduct formation in the liver will depend on the relative rates of oxidation of amodiaquine and reduction of its quinoneimine. This in turn may be a predisposing factor in the idiosyncratic hepatotoxicity associated with amodiaquine. Substitution of a fluorine for the phenolic hydroxyl group in amodiaquine blocked bioactivation of the drug in vivo. Insertion of an N-hydroxyethyl function enabled partial clearance of amodiaquine and its deshydroxyfluoro analogue via O-glucuronidation and altered the balance between phase I oxidation and direct phase II conjugation of amodiaquine. Amodiaquine (AQ) metabolism to N-desethylamodiaquine (DEAQ) is the principal route of disposition in humans. Using human liver microsomes and two sets of recombinant human cytochrome P450 isoforms (from lymphoblastoids and yeast) /the authors/ performed studies to identify the CYP isoform(s) involved in the metabolism of AQ. CYP2C8 was the main hepatic isoform that cleared AQ and catalyzed the formation of DEAQ. The extrahepatic P450s, 1A1 and 1B1, also cleared AQ and catalyzed the formation of an unknown metabolite M2. The K(m) and V(max) values for AQ N-desethylation were 1.2 microM and 2.6 pmol/min/pmol of CYP2C8 for recombinant CYP2C8, and 2.4 microM and 1462 pmol/min/mg of protein for human liver microsomes (HLMs), respectively. Relative contribution of CYP2C8 in the formation of DEAQ was estimated at 100% using the relative activity factor method. Correlation analyses between AQ metabolism and the activities of eight hepatic P450s were made on 10 different HLM samples. Both the formation of DEAQ and the clearance of AQ showed excellent correlations (r(2) = 0.98 and 0.95) with 6alpha-hydroxylation of paclitaxel, a marker substrate for CYP2C8. The inhibition of DEAQ formation by quercetin was competitive with K(i) values of 1.96 for CYP2C8 and 1.56 microM for HLMs. Docking of AQ into the active site homology models of the CYP2C isoforms showed favorable interactions with CYP2C8, which supported the likelihood of an N-desethylation reaction. These data show that CYP2C8 is the main hepatic isoform responsible for the metabolism of AQ. The specificity, high affinity, and high turnover make AQ desethylation an excellent marker reaction for CYP2C8 activity. Biological Half-Life 5.2 ± 1.7 (range 0.4 to 5.5) minutes Amodiaquine 600 mg was given by mouth, the apparent terminal half-life of amodiaquine was 5.2 + 1.7 (range 0.4 to 5.5) minutes and the geometric mean of the estimated elimination phase half-lives was 2.1 (range 0.5 to 5.7) hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Amodiaquine has been linked to serum aminotransferase elevations in a small proportion of patients (1%). More importantly, there have been multiple reports of idiosyncratic acute liver injury due to amodiaquine. The onset of injury is usually within 1 to 4 months and is often associated with agranulocytosis. The pattern of serum enzyme elevations is most frequently hepatocellular, and symptoms resembling acute viral hepatitis are typical. Features of hypersensitivity are uncommon, as are autoantibodies. The hepatitis can be severe, and several fatal instances or cases requiring emergency liver transplantation have been reported. The frequency of serious hepatic injury is estimated to be ~1:15,000. Because of the risks of agranulocytosis and liver injury, amodiaquine is no longer recommended for use as prophylaxis against malaria and is used largely for therapy in endemic areas outside of the United States. General recommendations on the therapy of malaria including specific details on diagnosis, management, drug dosage and safety are available at the CDC website: http://www.cdc.gov/malaria/. Likelihood score: A (well established cause of clinically apparent liver injury). Interactions Since magnesium trisilicate and kaolin are known to decrease the gastrointestinal absorption of chloroquine when administered simultaneously, it is likely that this also follows for amodiaquine. Concomitant administration of chloroquine at recommended doses for malaria suppression of chemoprophylaxis during pre-exposure prophylaxis of rabies with intra-dermally administered rabies vaccine may interfere with the antibody response to the vaccine. However, the clinical significance of this interaction remains to be clearly established but should be considered and may have relevance in the case of amodiaquine. Concomitant use with other antimalarials should be avoided and regular laboratory investigations should be performed to assure that blood values and liver function tests remain within normal limits. |
| 参考文献 |
[1]. Chun-Hyung Kim, et al. Nuclear receptor Nurr1 agonists enhance its dual functions and improve behavioral deficits in an animal model of Parkinson's disease. Proc Natl Acad Sci U S A. 2015 Jul 14;112(28):8756-61.
[2]. Keita Kinoshita, et al. A Nurr1 agonist amodiaquine attenuates inflammatory events and neurological deficits in a mouse model of intracerebral hemorrhage. J Neuroimmunol. 2019 May 15;330:48-54. [3]. Akira Yokoyama, et al. Effect of amodiaquine, a histamine N-methyltransferase inhibitor, on, Propionibacterium acnes and lipopolysaccharide-induced hepatitis in mice. Eur J Pharmacol. 2007 Mar 8;558(1-3):179-84. [4]. M T HOEKENGA. The treatment of acute malaria with single oral doses of amodiaquin, chloroquine, hydroxychloroquine and pyrimethamine. Am J Trop Med Hyg. 1954 Sep;3(5):833-8. |
| 其他信息 |
Therapeutic Uses
THERAP CAT: Antimalarial There are very few recent data on the in vivo susceptibility of P. ovale and P. malariae to antimalarials. Both species are regarded as very sensitive to chloroquine, although there is a single recent report of chloroquine resistance in P. malariae. Experience indicates that P. ovale and P. malariae are also susceptible to amodiaquine, mefloquine and the artemisinin derivatives. Summary of recommendations on the treatment of uncomplicated vivax malaria: Amodiaquine (30 mg base/kg bw divided over 3 days as 10 mg/kg bw single daily doses) combined with primaquine should be given for chloroquine-resistant vivax malaria. /Indicated/ for /the/ treatment of acute malarial attacks in non-immune subjects. It is at least as effective as chloroquine, and is effective against some chloroquine-resistant strains, although resistance to amodiaquine has been reported. Drug Warnings Agranulocytosis Associated with the Use of Amodiaquine for Malaria Prophylaxis Seven cases of agranulocytosis associated with the use of amodiaquine (Camoquine) among British travelers have recently been reported (1). Sixteen additional cases of agranulocytosis from Western Europe associated with the use of amodiaquine have recently been reported to the drug manufacturer, and two U.S. cases have been reported to CDC. Twenty-three of these 25 cases occurred in 1985 or 1986, and seven are reported to have been fatal. Among 20 cases for which the duration of amodiaquine prophylaxis is known, usage ranged from 3 weeks to 24 weeks. In all but four of the 25 cases, amodiaquine was used at the appropriate dosage (adults 400 mg base per week) for prophylaxis. Fourteen of the patients are known to have used another antimalarial drug concurrently for prophylaxis ... It is now apparent that any possible prophylactic advantage that amodiaquine may afford is not justified by the possible risk of agranulocytosis associated with the use of the drug. CDC, therefore, no longer recommends that amodiaquine be used for prophylaxis. Because amodiaquine may concentrate in the liver, the drug should be used with caution in patients with hepatic disease or alcoholism, and in patients receiving hepatotoxic drugs. Children are especially sensitive to 4-aminoquinoline derivatives. Because of the narrow margin between the therapeutic and toxic concentrations in children, amodiaquine should not be administered parenterally in this age group. Amodiaquine is contraindicated in patients who are hypersensitive /to 4-aminoquinoline derivatives/. For more Drug Warnings (Complete) data for AMODIAQUINE (14 total), please visit the HSDB record page. Pharmacodynamics Amodiaquine, a 4-aminoquinoline similar to chloroquine in structure and activity, has been used as both an antimalarial and an anti-inflammatory agent for more than 40 years. Amodiaquine is at least as effective as chloroquine, and is effective against some chloroquine-resistant strains, although resistance to amodiaquine has been reported. The mode of action of amodiaquine has not yet been determined. 4-Aminoquinolines depress cardiac muscle, impair cardiac conductivity, and produce vasodilatation with resultant hypotension. They depress respiration and cause diplopia, dizziness and nausea. |
| 精确质量 |
355.145
|
|---|---|
| CAS号 |
86-42-0
|
| 相关CAS号 |
Amodiaquine dihydrochloride dihydrate;6398-98-7;Amodiaquine-d10;1189449-70-4;Amodiaquine dihydrochloride;69-44-3
|
| PubChem CID |
2165
|
| 外观&性状 |
Crystals from absolute ethanol
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
478.0±45.0 °C at 760 mmHg
|
| 熔点 |
208°C
|
| 闪点 |
242.9±28.7 °C
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
| 折射率 |
1.669
|
| LogP |
4.77
|
| tPSA |
48.39
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
25
|
| 分子复杂度/Complexity |
406
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
OVCDSSHSILBFBN-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23)
|
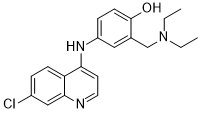
| 化学名 |
4-[(7-chloroquinolin-4-yl)amino]-2-(diethylaminomethyl)phenol
|
| 别名 |
Camochin Camoquin Camoquinal Camoquine Flavoquine Miaquin NSC 13453 SN-10751
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~66.67 mg/mL (~187.35 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02741024 | COMPLETED | Drug: Amodiaquine-Artesunate (ASAQ) Drug: Artemether-Lumefantrine (AL) |
Malaria | Medecins Sans Frontieres, Netherlands | 2013-10 | Phase 4 |
| NCT01704508 | COMPLETED | Drug: Artemether-lumefantrine Drug: Dihydroartemisinin-piperaquine |
Malaria | Bandim Health Project | 2012-11 | Phase 4 |
| NCT02627456 | COMPLETED | Biological: PfSPZ Vaccine Biological: PfSPZ Challenge Material Drug: PBS and HSA Diluent |
Malaria | National Institute of Allergy and Infectious Diseases (NIAID) | 2015-12-09 | Phase 1 |
|