| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|

| 靶点 |
- Epidermal growth factor receptor (EGFR) (Ki values in the low nanomolar range for various EGFR mutants)
- ERBB2 (also known as HER2) - ERBB family members in general, as it is a pan - ERBB inhibitor [1] |
|---|---|
| 体外研究 (In Vitro) |
- 在体外有效抑制EGFR激活突变以及EGFR T790M耐药突变的活性。在基于细胞的实验中,它对EGFR突变体的磷酸化表现出显著抑制作用,阻断了与细胞增殖相关的下游信号通路,如MAPK和AKT通路。例如,在具有EGFR激活突变或T790M耐药突变的肺癌细胞系中,达可替尼处理导致细胞活力和增殖呈剂量依赖性下降 [1]
- 抑制对Anti - Human HER2和GW572016耐药的HER2扩增乳腺癌细胞系的增殖。在HER2扩增的乳腺癌细胞系中,达可替尼处理以剂量依赖的方式抑制细胞生长。它还能有效降低HER2及其下游信号分子(如AKT和ERK)的磷酸化水平,这些分子对细胞存活和增殖至关重要 [2] |
| 体内研究 (In Vivo) |
- 在对ZD1839(吉非替尼)耐药的具有EGFR和ERBB2突变的肺癌模型中显示出有效性。在具有EGFR激活突变或ERBB2突变的肺癌异种移植小鼠模型中,口服给予达可替尼导致肿瘤显著消退。与对照组相比,肿瘤生长受到抑制,小鼠的总生存期得到改善。该药物通过抑制肿瘤组织中EGFR和ERBB2的激活,减少促存活和促增殖因子的产生来实现这一效果 [1]
|
| 细胞实验 |
- 对于肺癌细胞系:将具有不同EGFR突变(如激活突变和T790M耐药突变)的肺癌细胞系培养在合适的生长培养基中。将细胞以特定密度接种于96孔板中。过夜孵育使细胞贴壁后,将不同浓度(范围从低纳摩尔到微摩尔水平)的达可替尼加入孔中。在一定的孵育时间(通常为48 - 72小时)后,使用如MTT法或基于ATP的细胞活力测定法测量细胞活力。根据这些测定获得的吸光度或发光值计算细胞增殖的抑制率,并生成剂量 - 反应曲线以确定IC50值 [1]
- 对于乳腺癌细胞系:将HER2扩增的乳腺癌细胞系培养在合适的培养基中。将细胞铺在96孔板中。细胞贴壁后,加入不同浓度的达可替尼。随时间监测细胞生长情况,例如,在特定时间点(如24、48和72小时)使用细胞计数器计数细胞数量,或使用如XTT法测量细胞的代谢活性。在达可替尼处理后,还对细胞裂解物进行蛋白质免疫印迹分析。通过在合适的裂解缓冲液中裂解细胞来制备细胞裂解物。蛋白质通过SDS - PAGE电泳分离,然后转移到硝酸纤维素膜上。用针对HER2、磷酸化HER2、AKT、磷酸化AKT、ERK和磷酸化ERK的抗体对膜进行检测,以评估达可替尼对HER2信号通路的影响 [2] |
| 动物实验 |
- In the lung cancer xenograft models: Human lung cancer cell lines with EGFR or ERBB2 mutations were subcutaneously injected into the flanks of nude mice. Once the tumors reached a certain volume (usually around 100 - 200 mm³), the mice were randomly divided into treatment and control groups. Dacomitinib was formulated in a suitable vehicle (such as a mixture of DMSO and PEG 400 in saline). The drug was administered orally to the treatment group mice at a specific dose (e.g., 10 - 50 mg/kg) once daily for a defined period (usually 2 - 4 weeks). Tumor volumes were measured twice a week using calipers, and the body weights of the mice were also monitored. Tumor volume was calculated using the formula: volume = length × width² × 0.5. At the end of the treatment period, the mice were sacrificed, and tumors were excised for further analysis, such as immunohistochemistry to assess the expression of EGFR, ERBB2, and their phosphorylated forms [1]
|
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the clinical use of dacomitinib during breastfeeding. Because dacomitinib is 98% bound to plasma proteins, the amount in milk is likely to be low. However, because of its potential toxicity in the breastfed infant and its half-life of 70 hours, the manufacturer recommends that breastfeeding be discontinued during dacomitinib therapy and for at least 17 days after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| 参考文献 |
|
| 其他信息 |
- Dacomitinib is an irreversible pan - ERBB inhibitor. It binds covalently to nucleophilic cysteine residues in the catalytic domains of ERBB family members at the ATP - binding site, leading to irreversible inhibition of their tyrosine kinase activity. This inhibition blocks the downstream signaling cascades that are essential for cell proliferation, survival, and migration, making it a potential therapeutic agent for cancers with mutations and/or amplifications of ERBB family members [1]
Dacomitinib is a highly selective, orally bioavailable small-molecule inhibitor of the HER family of tyrosine kinases with potential antineoplastic activity. Dacomitinib specifically and irreversibly binds to and inhibits human Her-1, Her-2, and Her-4, resulting in the proliferation inhibition and apoptosis of tumor cells that overexpress these receptors. Drug Indication Vizimpro, as monotherapy, is indicated for the first-line treatment of adult patients with locally advanced or metastatic non small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) activating mutations. The use of targeted therapy in the management of epidermal growth factor receptor (EGFR)-mutated non-small-cell lung cancer is an important milestone in the management of advanced lung cancer. There are several generations of EGFR tyrosine kinase inhibitors available for clinical use. Dacomitinib is a second-generation irreversible EGFR tyrosine kinase inhibitor with early-phase clinical studies showing efficacy in non-small-cell lung cancer. In the recently published ARCHER 1050 phase III study, dacomitinib given at 45 mg/day orally was superior to gefitinib, a first-generation reversible EGFR tyrosine kinase inhibitor, in improving both progression-free survival and overall survival when given as first-line therapy. There is no prospective evidence to support the use of dacomitinib as subsequent therapy in patients previously treated with chemotherapy or a first-generation EGFR tyrosine kinase inhibitor such as gefitinib and erlotinib. Dacomitinib has not demonstrated any benefit in unselected patients with non-small-cell lung cancer, and its use should be limited to those with known EGFR-sensitizing mutations. Dacomitinib is associated with increased toxicities of diarrhea, rash, stomatitis, and paronychia compared with first-generation EGFR inhibitors. Global quality of life was maintained when assessed in phase III studies. Overall, dacomitinib is an important first- line agent in EGFR-mutated non-small-cell lung cancer in otherwise fit patients whose toxicities can be well managed.[3] |
| 分子式 |
C24H27CLFN5O3
|
|---|---|
| 分子量 |
487.95428776741
|
| 精确质量 |
487.178
|
| 元素分析 |
C, 59.08; H, 5.58; Cl, 7.26; F, 3.89; N, 14.35; O, 9.84
|
| CAS号 |
1042385-75-0
|
| 相关CAS号 |
Dacomitinib;1110813-31-4;Dacomitinib-d10 dihydrochloride;Dacomitinib-d10
|
| PubChem CID |
70693519
|
| 外观&性状 |
White to light yellow solid powder
|
| LogP |
5.751
|
| tPSA |
92.1
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
665
|
| 定义原子立体中心数目 |
0
|
| SMILES |
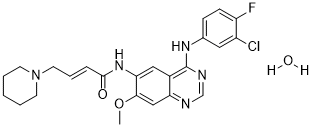
ClC1=C(C=CC(=C1)NC1=C2C(C=C(C(=C2)NC(/C=C/CN2CCCCC2)=O)OC)=NC=N1)F.O
|
| InChi Key |
BSPLGGCPNTZPIH-IPZCTEOASA-N
|
| InChi Code |
InChI=1S/C24H25ClFN5O2.H2O/c1-33-22-14-20-17(24(28-15-27-20)29-16-7-8-19(26)18(25)12-16)13-21(22)30-23(32)6-5-11-31-9-3-2-4-10-31;/h5-8,12-15H,2-4,9-11H2,1H3,(H,30,32)(H,27,28,29);1H2/b6-5+;
|
| 化学名 |
(E)-N-[4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolin-6-yl]-4-piperidin-1-ylbut-2-enamide;hydrate
|
| 别名 |
Dacomitinib; Dacomitinib monohydrate; PF-00299804; PF00299804; PF 00299804; PF299804; PF-299804; PF 299804; PF-299; PF299; PF 299; PF-00299804-03; Vizimpro
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0494 mL | 10.2470 mL | 20.4939 mL | |
| 5 mM | 0.4099 mL | 2.0494 mL | 4.0988 mL | |
| 10 mM | 0.2049 mL | 1.0247 mL | 2.0494 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04155541 | Recruiting | Drug: dacomitinib hydrate | EGFR Mutation-positive Inoperable or Reccrent NSCLC |
Pfizer | January 24, 2020 |