| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
Ileal bile acid transporter/IBAT (IC50 = 0.53 nM); IL-6; TNF-α/β
|
|---|---|
| 体外研究 (In Vitro) |
Elobixbat(原A3309)可减少BA在回肠末端的重吸收,导致粪便中BA排泄量增加,结肠中BA浓度升高,从而促进水和电解质向结肠的分泌,改善肠道动力,缓解结肠运输[2]。
Elobixbat(原A3309)是一种一流的回肠胆汁酸转运蛋白(IBAT)抑制剂,用于治疗慢性特发性便秘(CIC;Syn功能性便秘)。多达25%的人口受CIC影响;多达一半的人对目前的治疗方法不满意。目前对安全有效的CIC治疗药物的需求还没有得到满足。依洛比昔巴提供了一种新的方法,通过IBAT抑制,增强胆汁酸向结肠的输送,治疗慢性便秘。[1] |
| 体内研究 (In Vivo) |
Elobixibat治疗可降低血清BA,增加粪便BA浓度,改善肝脏炎症和纤维化。它还降低了肝脏和mln中促炎因子的表达以及肝脏中转化生长因子-β的表达。最后,elobixibat使肠道紧密连接蛋白水平和肠道微生物群组成正常化。
结论:elobixibat改善mcd喂养小鼠NASH相关组织病理学,降低细胞因子表达,并使肠道微生物组成正常化,这表明它可能是治疗NASH的有希望的候选药物。[3]
两组均出现肝脏脂肪堆积和纤维化,两组间差异无统计学意义。然而,使用elobixibat的小鼠肝脏肿瘤较少。血清总胆汁酸水平,包括游离胆汁酸、牛磺酸偶联胆汁酸、糖偶联胆汁酸和牛磺酸-α/β-胆酸,在elobixibat治疗后显著降低。伊洛比西巴治疗组粪便中革兰氏阳性菌比例(5.4%)显著低于未治疗组(33.7%)。 结论:elobixibat通过抑制胆汁酸重吸收,降低血清和肝脏中总胆汁酸和原胆汁酸水平来抑制肿瘤生长。此外,胆汁酸在结肠中的存在可能导致革兰氏阳性菌的比例显著减少,可能导致继发性胆汁酸合成减少[2]。 药效学研究表明,它能加速CIC患者的结肠运输,增加大便频率,使大便粘稠度变松,缓解便秘相关症状。这些有益效果至少可以连续治疗8周。elobixibat具有最小的吸收和较低的全身生物利用度,通常耐受性良好,并且可能通过胆汁酸消耗提供改善血清脂质谱的额外益处。[1] |
| 动物实验 |
Three-week-old male C57BL/6J mice were randomly divided into two groups (Fig. 1a): (1) CDHF diet + DEN (control group) and (2) CDHF diet + DEN + elobixibat (elobixibat group) groups. The mice received a single intraperitoneal injection of 25-mg/kg DEN at 3 weeks of age. Then, they were fed a standard diet until they reached 8 weeks of age. For the next 20 weeks, mice in the control group were fed a CDHF diet (60 kcal% fat), while those in the elobixibat group were fed a CDHF diet mixed with elobixibat. The animals were housed in a controlled environment (temperature 23 ± 1 °C, humidity 50 ± 10%, 12-h light/dark cycle) at the animal facility with unlimited access to food and water.[2]
Dose setting for elobixibat[2] Elobixibat was calculated to be 0.27 mg/kg/day. This study used animals (mean body weight of 23 g) based on the previously published data. The 50% inhibitory concentration of human IBAT is 0.53 nmol/L, and that of mouse IBAT is 0.13 nmol/L. Therefore, the inhibitory activity is four times higher in mice than in humans. The concentration of elobixibat in mice at 70% effective dose is 2.7 mg/kg; while at 50% effective dose, it is 0.27 (70% × [0.023/60])0.33 = 2.23 mg/kg; this would be 110 mg/day for a 50-kg human, 11 times the amount normally used. Therefore, we set our effective capacity at 50%: 0.27 (50%) × [0.023/60])0.33 = 0.223 mg/kg. A CDHF + elobixibat diet containing 3 mg elobixibat per kg of CDHF diet was created and used based on the mean expected body weight and expected food intake.[2] Elobixibat (1.2 mg/kg/day) was administered for the final 4 weeks of this period (elobixibat group). At the end of the study period, the mice were euthanized by inhalation of carbon dioxide.[2] Doses of 0.2, 0.6, or 1.2 mg/kg of elobixibat were administered for 4 weeks, 5 days per week, by oral gavage. Both the control group and the MCD-NASH group were administered PBS on the same schedule by oral gavage. There were no clear effects of 0.2 mg/kg or 0.6 mg/kg (data not shown), but beneficial effects were observed at the 1.2 mg/kg/day dose. This concentration is 4–6 times the dosage for human. According to “Drug Interview Form of Elobixibat”, elobixibat showed strong inhibitory activity to human IBAT, which was about four times more than that of mouse IBAT. Therefore, a dose of 1.2 mg/kg/day in mice is considered equivalent to 0.3 mg/kg/day in human. We evaluated the effects of this dose on the severity of NASH, cytokine production, the intestinal microbiota, and the intestinal TJs in the mice. No diarrhea was observed during the rearing period, and there was no difference in body weight between the NASH and elobixibat groups at the end of the experiment.[2] |
| 参考文献 | |
| 其他信息 |
Elobixibat has been used in trials studying the treatment and basic science of Dyslipidemia, Constipation, Chronic Constipation, Functional Constipation, and Chronic Idiopathic Constipation.
Drug Indication: Treatment of chronic constipation Elobixibat (formerly A3309) is a first-in-class ileal bile acid transporter (IBAT) inhibitor for treatment of chronic idiopathic constipation (CIC; syn functional constipation). CIC affects up to 25% of the general population; and up to a half are unsatisfied with current therapies. There is an unmet need for safe and effective drugs to treat CIC. Areas covered: The authors present: i) an overview of Phase II clinical trials of elobixibat in CIC, based on peer-reviewed literature and congress presentations and ii) an evaluation of the efficacy and mechanism of action of elobixibat in the treatment of CIC. Expert opinion: Elobixibat provides a novel approach to treat chronic constipation via IBAT inhibition with enhanced delivery of bile acids to the colon. Pharmacodynamic studies show that it accelerates colonic transit, increases stool frequency, loosens stool consistency and relieves constipation-related symptoms in CIC patients. These beneficial effects are maintained for a minimum of 8 consecutive weeks of treatment. With minimal absorption and low systemic bioavailability, elobixibat is generally well tolerated and may offer the added benefit of improving serum lipid profiles through bile acid depletion.[1] Elevated bile acid levels have been associated with liver tumors in fatty liver. Ileal bile acid transporter inhibitors may inhibit bile acid absorption in the distal ileum and increase bile acid levels in the colon, potentially decreasing the serum and hepatic bile acid levels. This study aimed to investigate the impact of these factors on liver tumor. Methods: C57BL/6J mice received a one-time intraperitoneal injection of 25-mg/kg diethylnitrosamine. They were fed a choline-deficient high-fat diet for 20 weeks starting from 8 weeks of age, with or without elobixiba[2] Recent studies have suggested that several types of toxic bile acids (BAs) are involved in the pathogenesis of non-alcoholic steatohepatitis (NASH). In the present study, we aimed to determine whether elobixibat, an ileal bile acid transporter (IBAT) inhibitor, would ameliorate NASH in mice. Methods: C57BL/6N mice were fed a methionine and choline-deficient (MCD) to induce NASH or standard diet as control for 8 weeks (n = 5 per group). The MCD diet-fed mice were administered elobixibat 5 days a week for 4 weeks by gavage (n = 5). The effects of the treatments on liver histopathology, proinflammatory cytokine concentrations, intestinal epithelial tight junctions, and the intestinal microbial composition were then assessed.[3] |
| 分子式 |
C36H47N3O8S2
|
|---|---|
| 分子量 |
713.9037
|
| 精确质量 |
713.28
|
| CAS号 |
1633824-78-8
|
| 相关CAS号 |
Elobixibat;439087-18-0
|
| PubChem CID |
121494007
|
| 外观&性状 |
White to off-white solid powder
|
| tPSA |
177Ų
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
16
|
| 重原子数目 |
49
|
| 分子复杂度/Complexity |
1140
|
| 定义原子立体中心数目 |
1
|
| SMILES |
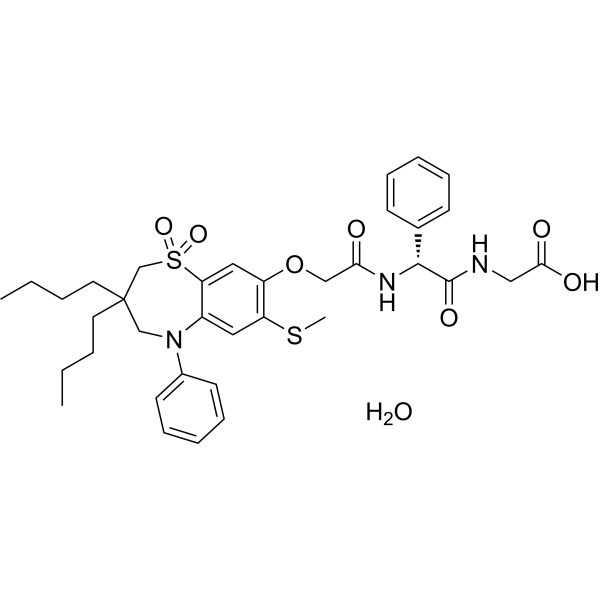
S1(C2=CC(=C(C=C2N(C2C=CC=CC=2)CC(CCCC)(CCCC)C1)SC)OCC(N[C@@H](C(NCC(=O)O)=O)C1C=CC=CC=1)=O)(=O)=O.O
|
| InChi Key |
VARDBGNECHECBX-MDYNBEAQSA-N
|
| InChi Code |
InChI=1S/C36H45N3O7S2.H2O/c1-4-6-18-36(19-7-5-2)24-39(27-16-12-9-13-17-27)28-20-30(47-3)29(21-31(28)48(44,45)25-36)46-23-32(40)38-34(26-14-10-8-11-15-26)35(43)37-22-33(41)42;/h8-17,20-21,34H,4-7,18-19,22-25H2,1-3H3,(H,37,43)(H,38,40)(H,41,42);1H2/t34-;/m1./s1
|
| 化学名 |
2-[[(2R)-2-[[2-[(3,3-dibutyl-7-methylsulfanyl-1,1-dioxo-5-phenyl-2,4-dihydro-1λ6,5-benzothiazepin-8-yl)oxy]acetyl]amino]-2-phenylacetyl]amino]acetic acid;hydrate
|
| 别名 |
elobixibat hydrate; Elobixibat monohydrate; Elobixibat hydrate [MI]; C8YTS56FNG; UNII-C8YTS56FNG; 1633824-78-8; Elobixibat hydrate (JAN); Glycine, (2R)-N-(2-((3,3-dibutyl-2,3,4,5-tetrahydro-7-(methylthio)-1,1-dioxido-5-phenyl-1,5-benzothiazepin-8-yl)oxy)acetyl)-2-phenylglycyl-, hydrate (1:1);
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4008 mL | 7.0038 mL | 14.0076 mL | |
| 5 mM | 0.2802 mL | 1.4008 mL | 2.8015 mL | |
| 10 mM | 0.1401 mL | 0.7004 mL | 1.4008 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。