| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
D1/D2 Receptor; PI3Kα (IC50 = 127 nM)
|
|---|---|
| 体外研究 (In Vitro) |
使用氟哌噻吨(2.5-40 μM;72 小时)治疗可剂量依赖性地降低肺癌细胞活力[3]。为了诱导肺癌细胞凋亡,给予氟哌噻吨 (2.5-40 μM) 24 小时 [3]。 Bcl-2 表达水平和 p-AKT 受氟哌噻吨 (2.5–15 μM;24 小时) 抑制 [3]。
|
| 体内研究 (In Vivo) |
鼻内注射氟哌噻吨(40 mg/kg,每天一次,持续 21 天)可防止裸鼠形成 A549 异种移植肿瘤 [3]。
氟哌噻吨抑制裸鼠A549异种移植物肿瘤生长[3] 最后,我们评估了氟哌噻吨对肺癌体内生长的影响。BALB/C裸鼠皮下注射A549细胞。接种后14天,肿瘤生长到50-80mm3的体积。将小鼠随机分为两组(每组6只),每天灌胃注射PBS(对照组)或氟哌噻吨(40mg/kg),持续21天。我们的结果显示,与赋形剂对照组相比,氟哌噻吨显著减少了肿瘤体积(p<0.05)(图55A)。氟哌噻吨还显著降低了64.1%的肿瘤重量(p<0.05)(图55B-C)。氟哌噻吨治疗对小鼠的平均体重没有实质性影响(图55D)。这些结果表明,氟哌噻吨是一种潜在的安全有效的口服抗癌药物,用于治疗癌症。 该研究调查了氟哌噻吨在治疗阴性症状方面与利培酮相比的非劣效性。此外,还探讨了氟哌噻吨对情绪和认知症状的影响。在一项随机、双盲、多中心研究中,144名以阴性症状为主的非急性精神分裂症患者接受了灵活剂量的氟哌噻吨(4-12mg/d)或利培酮(2-6mg/d)治疗,治疗时间长达25周。除了非劣效性分析外,还对PANSS进行了事后主成分分析(PCA)。关于阴性症状,氟哌噻吨被证明不亚于利培酮。这两种药物都改善了抑郁情绪,效果大小有利于氟哌噻吨。PCA建议采用五因素结构。认知因素的效应大小,氟哌噻吨高达0.74,利培酮高达0.80。两组的EPS评分均较低,帕金森病均有改善,但氟哌噻吨组的抗胆碱能药物处方频率明显更高,通常显示出明显更多的不良事件。结果表明,与利培酮相比,第一代抗精神病药物氟哌噻吨可改善慢性精神分裂症患者的阴性、情感和认知症状。进一步的研究应使用神经心理学性能测试来证实后者,并应调查耐受性是否会随着剂量范围的显著降低而提高[3]。 |
| 酶活实验 |
无细胞生化激酶抑制试验[2]
通过评估重组激酶PI3Kα对聚EY(4:1 Glu,Tyr)肽底物的磷酸化,在无细胞系统中检测了氟戊四醇对PI3Kα的抑制作用。根据制造商的说明,使用ADP-Glo激酶检测试剂盒评估重组激酶的抑制作用。简而言之,将不同浓度范围(1 nM-1µM)的氟戊四醇与4 ng重组激酶和0.2µg/mL聚EY底物在室温下孵育60分钟。然后,加入5µL ADP-Glo试剂,在室温下继续孵育40分钟。最后,加入10µL激酶检测试剂,使混合物在室温下温育30分钟,然后用GloMax 20/20光度计测量发光。 |
| 细胞实验 |
细胞活力测定[3]
细胞类型: A549、H661、SK-SEM-1 和 NCAL-H520 细胞 测试浓度: 2.5、5、 10、20 或 40 μM 孵育时间:72 小时 实验结果:显示 IC50 为 5.708 μM A549 和 H661 细胞分别为 6.374 μM 和 6.374 μM。 细胞凋亡分析[3] 细胞类型: A549 和 H661 细胞 测试浓度: 5、10、20 和 40 μM 孵育时间:24小时 实验结果:与阴性对照相比,A549和H661中早期凋亡细胞的百分比有所增加( p < 0.05)。以剂量依赖性方式诱导 PARP 和 caspase-3 裂解。 蛋白质印迹分析 [3] 细胞类型: H661 和 A549 细胞 测试浓度: 2.5、5、10 和 15 μM 孵育持续时间:24 小时 实验结果:以剂量依赖性方式降低 AKT 磷酸化水平和 Bcl-2 表达水平方式。 |
| 动物实验 |
Animal/Disease Models: BALB/C nude mice injected with A549 cells [3]
Doses: 40 mg/kg Route of Administration: gavage; 40 mg/kg; one time/day; 21 days Experimental Results: Compared with the vehicle control, the tumor volume diminished ( p<0.05), tumor weight diminished by 64.1% (p<0.05). A549 growth in nude mice[2] Male BALB/C nude mice of 5-6 weeks old were used. A549 cells (1×106/0.2 ml PBS per mice) were injected subcutaneously into the right flank of the mice. Seven days after inoculation, tumors grew to a volume of 80-100 mm3. The mice were randomly divided into two groups (six mice per group) and injected by intragastric injection administration (i.g.) every day for 21 days with either PBS (control group) or flupentixol (40 mg/kg in PBS). Tumor volumes were measured every 3-4 days after tumor appearance and calculated by the equation V=ab2/2 (a=longest axis; b=shortest axis). The mice were sacrificed on day 21 after treatment, and tumors were isolated and weighed. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following oral administration, flupentixol is readily absorbed from the gastrointestinal tract, with oral bioavailability of about 40%. Tmax ranges from three to eight hours. Steady-state plasma levels are achieved in about seven days and following once-daily oral administration of 5 mg flupentixol, the mean minimum steady-state level was about 1.7 ng/mL (3.9 nmol/L). From the site of intramuscular injection, esterified flupentixol diffuses slowly from the oil solution and is slowly released into the extracellular fluid and the circulation to be distributed to different tissues. Peak drug concentrations are reached between four and seven days following intramuscular injection. Intramuscularly administered flupentixol is detectable in the blood three weeks after injection and reaches steady-state concentrations after about three months of repeated administration. Fecal excretion is more predominant than renal excretion. In the feces, flupentixol is recovered in the feces mainly as the unchanged form, as well as its lipophilic metabolites, such as dealkyl-flupentixol. Flupentixol is recovered in the urine as the unchanged form as well as its hydrophilic sulfoxide and glucuronide metabolites. The apparent volume of distribution is about 14.1 L/kg. Following administration, the highest levels of flupentixol are found in the lungs, liver, and spleen. Lower concentrations of the drug are found in the blood and brain. Following oral administration, the mean systemic clearance is about 0.29 L/min. Metabolism / Metabolites Flupentixol is metabolized in the liver via sulfoxidation, dealkylation, and glucuronidation to form pharmacologically inactive metabolites. Flupentixol decanoate, the active ingredient in the intramuscular formulation, is hydrolyzed to flupentixol. Biological Half-Life The elimination half-life is about 35 hours following oral administration and three weeks following intramuscular administration. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Flupenthixol is not approved for marketing in the United States by the U.S. Food and Drug Administration, but is available in other countries. Limited information indicates that maternal oral doses of up to 4 mg daily or depot injections of 40 mg every 2 weeks produced low levels in milk and breastfed infants' serum, and caused no adverse developmental consequences. A safety scoring system finds flupenthixol possible to use cautiously during breastfeeding. Until more data are available, flupenthixol should be used with careful infant monitoring during breastfeeding. ◉ Effects in Breastfed Infants A woman took flupenthixol 1 mg and nortriptyline 100 mg daily during pregnancy and flupenthixol 4 mg and nortriptyline 125 mg daily immediately postpartum. She exclusively breastfed her infant. Over a 4-month period, the infant showed no signs of adverse drug effects and had normal motor development with a maternal dosage of flupenthixol 2 mg daily and nortriptyline 75 mg daily. ◉ Effects on Lactation and Breastmilk Flupenthixol can increase serum prolactin and has caused galactorrhea. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding Flupentixol is 99% bound to plasma proteins. |
| 参考文献 |
|
| 其他信息 |
Cis-flupenthixol is a flupenthixol in which the double bond adopts a cis-configuration. It has a role as a dopaminergic antagonist. It is a conjugate base of a cis-flupenthixol(2+).
Flupentixol is an antipsychotic drug of the thioxanthene group. It exists in two geometric isomers, the trans(E) and pharmacologically active cis(Z) forms. Flupentixol decanoate is one of the active ingredients found in injectable drug formulations: it is produced by esterification of cis(Z)‐flupentixol with decanoic acid. Flupentixol is an antagonist of both D1 and D2 dopamine receptors. Available as oral tablets or long-acting intramuscular injections, flupentixol is marketed under brand names such as Depixol and Fluanxol. It is approved for use in Canada and other countries around the world, but not in the US. It is used for the management of chronic schizophrenia in patients whose main manifestations do not include excitement, agitation or hyperactivity. It has been marketed to manage symptoms of depression in patients who may or may not exhibit signs of anxiety. In combination with [melitracen], flupentixol is used to manage symptoms of anxiety, depression, and asthenia. A thioxanthene neuroleptic that, unlike CHLORPROMAZINE, is claimed to have CNS-activating properties. It is used in the treatment of psychoses although not in excited or manic patients. (From Martindale, The Extra Pharmacopoeia, 30th ed, p595) Drug Indication Flupentixol is indicated for maintenance therapy of chronic schizophrenic patients whose main manifestations do not include excitement, agitation or hyperactivity. It is indicated for the management of depression in adult patients who may, or may not, also be showing signs of anxiety. Flupentixol in combination with [melitracen] is indicated to manage symptoms of anxiety, depression, and asthenia in adults. Mechanism of Action The mechanism of action of flupentixol is not completely understood. The antipsychotic actions are mainly thought to arise from cis(Z)-flupentixol, the active stereoisomer, acting as an antagonist at both dopamine D1 and D2 receptors with equal affinities. Schizophrenia is a mental illness characterized by positive (such as hallucinations and delusions) and negative (such as affect flattening and apathy) symptoms. While several neurotransmitter systems are implicated in the pathophysiologic processes leading to the development of symptoms, the dopamine and glutamate systems have been extensively studied. It is generally understood that positive symptoms of schizophrenia arise from a dysregulated striatal dopamine pathway, leading to hyperstimulation of D2 receptors. Many antipsychotic agents work by blocking D2 receptors as antagonists; similarly, cis(Z)-flupentixol, the active stereoisomer, is an antagonist at D2 receptors. However, there is now evidence that antipsychotic agents can work by blocking other dopamine receptor subtypes, such as D1, D3, or D4 receptors. One study showed that cis(Z)-flupentixol is an antagonist at both dopamine D1 and D2 receptors with equal affinities, and binds to D3 and D4 receptors with lower affinities. It also binds to alpha-1 adrenoceptors. Antidepressant effects of flupentixol are understood to be mediated by antagonism at 5-HT2A receptors, which are commonly downregulated following repeated antidepressant treatment. Flupentixol also binds to 5-HT2C receptors. Pharmacodynamics Flupentixol is an antipsychotic agent with anxiolytic and mild sedative actions. It exerts weak anticholinergic and adrenergic effects. It possesses antiemetic actions. As flupentixol works by antagonizing dopamine actions, it can cause extrapyramidal effects, mostly at doses greater than 10 mg. In clinical trials, flupentixol-induced extrapyramidal effects have been managed with anti-Parkinsonian drugs. Drug esterification in the intramuscular formulation of the drug results in slow release of the drug from the injection site and a prolonged duration of action. Flupentixol has been investigated for use in mild to moderate depression: compared to other antidepressant agents, flupentixol has a rapid onset of action, where antidepressive effects were observed within the first two to three days after administration. As with other antipsychotic agents, flupentixol can cause QTc prolongation and increase the risk of arrhythmias. In clinical trials, flupentixol was associated with the risk of cardiovascular disease, cerebrovascular adverse events, stroke, and venous thromboembolism. Flupentixol can elevate the levels of prolactin; however, the clinical significance of hyperprolactinemia caused by neuroleptic drugs is unclear. Long-term hyperprolactinemia, when associated with hypogonadism, may lead to decreased bone mineral density in both female and male subjects. Interestingly, recent studies show that flupentixol exhibits anti-tumour properties alone or synergistically with other anticancer drugs like gefitinib. One study demonstrated that _in vitro_, flupentixol docks to the ATP binding pocket of phosphatidylinositol 3-kinase (PI3K), a lipid kinase that activates signalling pathways that are often hyperactivated in some cancers. Flupentixol inhibited the PI3K/AKT pathway and survival of lung cancer cells _in vitro_ and _in vivo_. |
| 分子式 |
C23H25N2OF3S
|
|---|---|
| 分子量 |
434.5176
|
| 精确质量 |
434.164
|
| 元素分析 |
C, 63.58; H, 5.80; F, 13.12; N, 6.45; O, 3.68; S, 7.38
|
| CAS号 |
2709-56-0
|
| 相关CAS号 |
Flupentixol dihydrochloride;2413-38-9;cis-(Z)-Flupentixol dihydrochloride;51529-01-2; 51529-02-3 [(E)-Flupentixol]
|
| PubChem CID |
5281881
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.306g/cm3
|
| 沸点 |
554.7ºC at 760mmHg
|
| 熔点 |
233-234
|
| 闪点 |
289.3ºC
|
| 折射率 |
1.607
|
| LogP |
4.477
|
| tPSA |
52.01
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
592
|
| 定义原子立体中心数目 |
0
|
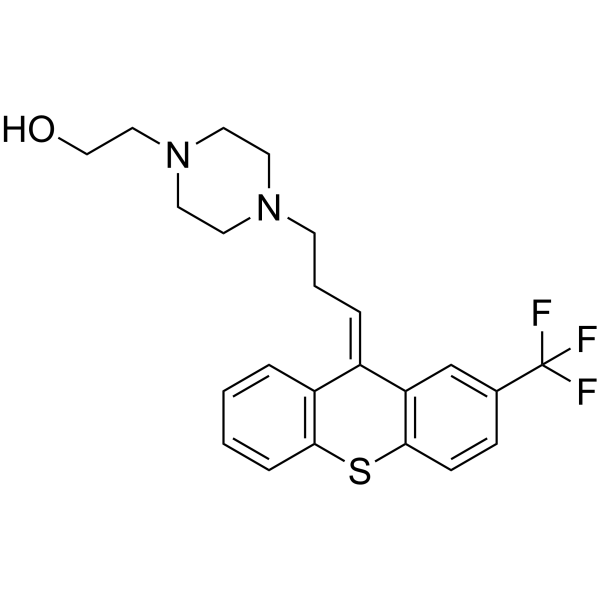
| SMILES |
OCCN1CCN(CC/C=C2\C3=CC=CC=C3SC3C=CC(=CC\2=3)C(F)(F)F)CC1
|
| InChi Key |
NJMYODHXAKYRHW-DVZOWYKESA-N
|
| InChi Code |
InChI=1S/C23H25F3N2OS/c24-23(25,26)17-7-8-22-20(16-17)18(19-4-1-2-6-21(19)30-22)5-3-9-27-10-12-28(13-11-27)14-15-29/h1-2,4-8,16,29H,3,9-15H2/b18-5-
|
| 化学名 |
2-[4-[(3Z)-3-[2-(trifluoromethyl)thioxanthen-9-ylidene]propyl]piperazin-1-yl]ethanol
|
| 别名 |
FLUPENTHIXOL; Flupentixol; trans-flupenthixol; trans-Flupentixol; (E)-Flupenthixol; 53772-85-3; beta-Flupenthixol; ...; 2709-56-0;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3014 mL | 11.5070 mL | 23.0139 mL | |
| 5 mM | 0.4603 mL | 2.3014 mL | 4.6028 mL | |
| 10 mM | 0.2301 mL | 1.1507 mL | 2.3014 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。