| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
p110α (IC50 = 0.5 μM); p110δ (IC50 = 0.57 μM); p110β (IC50 = 0.97 μM); human CK2 (IC50 = 98 nM); human CK2α2 (IC50 = 3.869 μM); DNA-PK (IC50 = 1.4 μM)
|
|---|---|
| 体外研究 (In Vitro) |
LY294002 盐酸盐(0-75 μM;24 和 48 小时)以剂量依赖性方式显着减少人鼻咽癌 CNE-2Z 细胞[4]。 LY294002 盐酸盐(0-75 μM;24 和 48 小时)剂量依赖性地增加 CNE-2Z 细胞的凋亡率[4]。在 CNE-2Z 细胞中,LY294002 盐酸盐 (10–75 μM) 显着降低 p-Akt (S473) 表达水平并增加 caspase-9 活性。不同浓度下总 Akt 蛋白水平没有变化 [4]。用 LY294002 盐酸盐(5、10、100 µM;持续 2 小时)处理可部分抑制溶血磷脂酸 (LPA)(20 µM;持续 4 小时)产生的 YAP 核转位,随后 p-AKT 水平降低[6]。
|
| 体内研究 (In Vivo) |
LY294002 盐酸盐(10、25、50、75 mg/kg;腹腔注射;每周两次;持续 4 周)以剂量依赖性方式显着降低平均 NPC 肿瘤负荷。 LY294002(10、25 mg/kg)降低肿瘤负荷的效果较低[4]。在 Sprague-Dawley 大鼠中,LY294002 盐酸盐(1.2 mg/kg ip;ip)持续 14 天可抑制瘦素(60 ug/kg)对精子的有害作用[5]。
|
| 酶活实验 |
LY294002 对 PI3K 的抑制作用是使用纯化的重组酶和 1μM ATP 在放射测定中测定的。在室温 (24oC) 下,激酶反应持续一小时,然后通过添加 PBS 停止。然后,通过拟合可变斜率 S 形剂量反应曲线来计算 IC50 值。激酶选择性筛选用于确定 CK2 和 GSK3β(糖原合酶激酶 3β)的抑制作用。在 10μM ATP 中,根据 Upstate 激酶组对 LY294002 进行评估。
|
| 细胞实验 |
细胞增殖测定
细胞类型: CNE-2Z 细胞[4] 测试浓度:0 μM、10 μM、25 μM、50 μM 和 75 μM 孵育持续时间:24小时和48小时 实验结果:以剂量依赖性方式减少CNE-2Z细胞。 细胞凋亡分析 细胞类型: CNE-2Z 细胞[4] 测试浓度: 0 μM、10 μM、25 μM、 50 μM 和 75 μM 孵育时间: 24 小时和 48 小时 实验结果: 剂量下诱导细胞凋亡率依赖方式。 蛋白质印迹分析 细胞类型: CNE-2Z 细胞[4] 测试浓度: 0 μM、10 μM、25 μM 、50 μM 和 75 μM 孵育时间:24 小时和 48 小时 实验结果:磷酸化 Akt (S473) 减少治疗组 CNE-2Z 细胞中 caspase-9 的表达水平显着上调。 |
| 动物实验 |
Animal/Disease Models: Athymic nude mice (6-8 weeks) with CNE-2Z xenograft[4]
Doses: 10 mg/kg, 25 mg/kg, 50 mg/kg, and 75 mg/kg Route of Administration: IP; twice weekly, for 4 weeks Experimental Results: Mean Nasopharyngeal carcinoma (NPC) tumor burden was remarkably decreased in a dose-dependent manner. |
| 参考文献 |
|
| 其他信息 |
This research's purpose was to explore the existence of vasculogenic mimicry (VM) in both 3-D matrices of Panc-1 cells in vitro and orthotopic Panc-1 xenografts in vivo and to test the hypothesis that PI3K inhibitor LY294002 and gemcitabine hydrochloride would offer clear treatment benefit when integrated into ionizing radiation (IR) therapeutic regimens for treatment of pancreatic cancer. We explored the existence of VM in both 3-D matrices of Panc-1 cells and orthotopic Panc-1 xenografts. We subsequently investigated the activation of the PI3K/MMPs/Ln-5γ2 signaling pathway in response to IR. LY294002 and gemcitabine hydrochloride were then evaluated for their radiosensitizing effect solely and in combination. We found that VM existed in both 3-D matrices of Panc-1 cells in vitro and orthotopic Panc-1 xenografts in vivo. The expressions of p-Akt and MMP- 2 were found to increase in response to IR. LY294002 and gemcitabine hydrochloride combined with IR better inhibited cell migration, VM formation and MMP-2 mRNA expression of Panc-1 cells in vitro, and we also proved that the novel therapeutic regimen better inhibited tumor growth, tumor metastasis and VM formation of orthotopic Panc-1 xenografts by suppressing the PI3K/MMPs/Ln-5γ2 signaling pathway in vivo. Our present study is among the first to prove the VM formation in orthotopic Panc-1 xenografts. Furthermore, our current study is also among the first to provide preliminary evidence for the use of the novel therapeutic regimen LY294002 and gemcitabine hydrochloride combined with IR for treatment of pancreatic cancer.[1]
The PI3Ks (phosphatidylinositol 3-kinases) regulate cellular signalling networks that are involved in processes linked to the survival, growth, proliferation, metabolism and specialized differentiated functions of cells. The subversion of this network is common in cancer and has also been linked to disorders of inflammation. The elucidation of the physiological function of PI3K has come from pharmacological studies, which use the enzyme inhibitors Wortmannin and LY294002, and from PI3K genetic knockout models of the effects of loss of PI3K function. Several reports have shown that LY294002 is not exclusively selective for the PI3Ks, and could in fact act on other lipid kinases and additional apparently unrelated proteins. Since this inhibitor still remains a drug of choice in numerous PI3K studies (over 500 in the last year), it is important to establish the precise specificity of this compound. We report here the use of a chemical proteomic strategy in which an analogue of LY294002, PI828, was immobilized onto epoxy-activated Sepharose beads. This affinity material was then used as a bait to fish-out potential protein targets from cellular extracts. Proteins with high affinity for immobilized PI828 were separated by one-dimensional gel electrophoresis and identified by liquid chromatography-tandem MS. The present study reveals that LY294002 not only binds to class I PI3Ks and other PI3K-related kinases, but also to novel targets seemingly unrelated to the PI3K family.[3] Background: To evaluate whether PI3K/Akt pathway could effect on apoptosis and its mechanism in nasopharyngeal carcinoma cells. Methods: The activation of the PI3K/Akt and its effect on CNE-2Z cells in vivo and in vitro was investigated by MTT assay, flow cytometry, western blot, ELISA, terminal deoxyribonucleotide transferase-mediated nick-end labeling assays (TUNEL), and immunohistochemical analyses, using PI3K inhibitor, LY294002. Results: The results showed that LY294002 inhibited the phosphorylating of Akt (S473), cell proliferation, and induced apoptosis in CNE-2Z cells. However, our experiment results also demonstrated that apoptosis-induced LY294002 was directly regulated by caspase-9 activation pathway. Conclusion: These data suggested that PI3K inhibitor, LY294002, induced apoptosis by caspase-9 activation pathway and might be as a potentially useful target for therapeutic intervention in nasopharyngeal carcinoma patients.[4] |
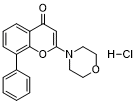
| 分子式 |
C19H17NO3.HCL
|
|---|---|
| 分子量 |
343.80412
|
| 精确质量 |
343.098
|
| 元素分析 |
C, 66.38; H, 5.28; Cl, 10.31; N, 4.07; O, 13.96
|
| CAS号 |
934389-88-5
|
| 相关CAS号 |
LY294002;154447-36-6
|
| PubChem CID |
11957589
|
| 外观&性状 |
Solid powder
|
| LogP |
4.163
|
| tPSA |
42.68
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
463
|
| 定义原子立体中心数目 |
0
|
| SMILES |
Cl.O=C1C2C=CC=C(C=2OC(N2CCOCC2)=C1)C1C=CC=CC=1
|
| InChi Key |
OQZQSRICUOWBLW-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H17NO3.ClH/c21-17-13-18(20-9-11-22-12-10-20)23-19-15(7-4-8-16(17)19)14-5-2-1-3-6-14;/h1-8,13H,9-12H2;1H
|
| 化学名 |
2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one hydrochloride
|
| 别名 |
LY-294002 HCl; LY294002 HCl;LY294002 hydrochloride; LY 294002 hydrochloride; LY 294002 HCl
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
Typically soluble in DMSO (e.g. > 10 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9087 mL | 14.5433 mL | 29.0867 mL | |
| 5 mM | 0.5817 mL | 2.9087 mL | 5.8173 mL | |
| 10 mM | 0.2909 mL | 1.4543 mL | 2.9087 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。