| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 体内研究 (In Vivo) |
纳洛酮(2.0 mg/kg,连续输注 1.7 mg/kg/h)可显着改善大鼠的神经行为结果,这种效果可持续长达 4 周。纳洛酮治疗会导致平均动脉血压 (MAP) 中度、非显着升高 [1]。纳洛酮(0.4 mg/kg)可以改善大鼠的记忆力并抑制ACTH和肾上腺素的遗忘作用[2]。纳洛酮给药以剂量相关的方式降低猫首次破伤风的强度。纳洛酮(5 或 10 mg/kg,静脉注射)随后续剂量的吗啡产生减少的 PTP 抑制,但对最大抽搐抑制没有影响 [3]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
An intranasal dose of naloxone is 42-47% bioavailable. An 8 mg dose of nasal naloxone reaches a Cmax of 12.3-12.8 ng/mL, with a Tmax of 0.25 hours, and an AUC of 16.7-19.0 h\*ng/mL. A 0.4 mg intramuscular dose reaches a Cmax of 0.876-0.910 ng/mL, with a Tmax of 0.25 hours, and an AUC of 1.94-1.95 h\*ng/mL. A 2 mg intravenous dose reaches a Cmax of 26.2 ng/mL with an AUC of 12.8 h\*ng/mL. After oral or intravenous administration, naloxone is 25-40% eliminated in the urine within 6 hours, 50% in 24 hours, and 60-70% in 72 hours. The metabolites naloxone-3-glucuronide, noroxymorphone, and naloxol are all detected in the urine. The volume of distribution of naloxone is 200 L. Naloxone distributes into tissues rapidly. It can also cross the placenta and blood-brain barrier. The clearance of naloxone is 2500 L/day. Naloxone is distributed rapidly throughout the body with high levels found in the brain, kidneys, spleen, skeletal muscle, lung, and heart. The drug also readily crosses the placenta. Naloxone is only minimally absorbed when given orally as it is rapidly destroyed in the GI tract. Much higher doses are required if using this route of administration for any pharmacologic effect. When given IV, naloxone has a very rapid onset of action (usually 1-2 minutes). If given IM, the drug generally has an onset of action with 5 minutes of administration. The duration of action usually persists from 45-90 minutes, but may act for up to 3 hours. Naloxone is rapidly inactivated following oral administration. Although the drug is effective orally, doses much larger than those required for parenteral administration are required for complete antagonism. In one study, a single 3-g oral dose of naloxone hydrochloride was required to effectively antagonize the effects of 50 mg of heroin for 24 hours. Naloxone has an onset of action within 1-2 minutes following iv administration and within 2-5 minutes following subcutaneous or im administration. The duration of action depends on the dose and route of administration and is more prolonged following im administration than after iv administration. Following administration of 35 or 70 ug of naloxone hydrochloride into the umbilical vein in neonates in one study, peak plasma naloxone concentrations occurred within 40 minutes and were 4-5.4 ng/mL and 9.2-20.2 ng/mL, respectively. After im administration of 0.2 mg to neonates in the same study, peak plasma naloxone concentrations of 11.3-34.7 ng/mL occurred within 0.5-2 hours. For more Absorption, Distribution and Excretion (Complete) data for NALOXONE (14 total), please visit the HSDB record page. Metabolism / Metabolites Naloxone primarily undergoes glucuronidation to form naloxone-3-glucuronide. Naloxone is also N-dealkylated to noroxymorphone or undergoes 6-keto reduction to naloxol. Naloxone is rapidly metabolized in the liver, principally by conjugation with glucuronic acid. The major metabolite is naloxone-3-glucuronide. Naloxone also undergoes N-dealkylation and reduction of the 6-keto group followed by conjugation. Yields N-allyl-7,8-dihydro-14-hydroxynormorphine, 7,8-dihydro-14-hydroxynormorphinone in man; Weinstein, SH, Pfeffer, M, Schor, JM, Indindoli, L, & Mintz, M, J Pharm Sci, 60, 1567 (1971). Yields naloxone-3-beta-d-glucuronide in man; Fujimoto, JM, J Pharmac Exp Ther, 168, 180(1969). /From table/ ... Oxidative N-deallylation, redn of 6-keto-group, and glucuronidation occur in man. ... Naloxone-3-glucuronide (major), 3-sulfate (minor), naloxol and conjugated naloxol (minor), 7,8-dihydro-14-hydroxynormorphine, 7,8-dihydro-14-hydroxynormorphine and their conjugates were shown to be the metabolites of naloxone. In addition, tentative evidence was obtained for two polar hydroxylated metabolites (with hydroxylation presumably in the 17-side chain or in position 2 of the aromatic nucleus). 7,8-Dihydro-14-hydroxynormorphinone and 2-polar metabolites were also observed in brain. ... Biological Half-Life The mean half life of naloxone hydrochloride is 1.8-2.7 hours intranasally, 1.4 hours intramuscularly, and 1.2 hours intravenously. In neonates, the mean half life is 3.1 ± 0.5 hours. The mean plasma half-life of naloxone was 1.28 hours following IM or subcutaneous injection of naloxone hydrochloride using an auto-injector, compared with 1.36 hours following IM or subcutaneous injection using a standard syringe. The half-life of naloxone has been reported to be 30-81 minutes in adults and about 3 hours in neonates. Plasma naloxone levels were determined by RIA over a period of 6--36 hr in three groups of neonates, (1) those given 35 microgram iv (n = 6), (2) those given 70 microgram iv (n = 6) and (3) those given 200 microgram im (n = 17) naloxone HCl within 1 min of birth. After intravenous administration of 35 and 70 microgram of naloxone peak levels of 4--15 ng/mL and 9--20 ng/mL respectively were reached in 5--40 min and the mean plasma half-life after both doses was 3.1 +/- 0.5 hr. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Naloxone is composed of crystals. Naloxone hydrochloride is used for the complete or partial reversal of opioid depression, including respiratory depression, induced by natural and synthetic opioids (both human and veterinary cases). It may also be useful as an adjunctive agent to increase blood pressure in the management of septic shock. A formulation for wildlife use (Trexonil) is more concentrated and used to reverse tranquilization in wild animals. HUMAN EXPOSURE AND SYMPTOMS: Adverse effects associated with naloxone use have included seizures, severe hypertension, and hypotension and/or bradycardia. A total of 1.2 mg administered intravenously as 0.2, 0.4, and 0.6 mg at 11, 22, and 33 minutes respectively to nonaddicts caused miosis, decreased core temperature, and systolic blood pressure. Naloxone-induced acute pulmonary edema is an extremely rare but lethal complication. Endogenous opioids appear to regulate blood pressure in some hypertensive patients and opiate antagonists such as naloxone must be administered with caution to these individuals. Moderate increases in respiratory rate, heart rate, and blood pressure occur after naloxone administration to children, but development of more serious complications is rare. Naloxone was weakly positive in the in vitro human lymphocyte chromosome aberration test. Naloxone may affect some functions of the immune system in humans, but its action is transient. ANIMAL STUDIES: The injection of naloxone into the medial septal nucleus of rats produced a marked increase in hippocampal ACh release in a dose-dependent manner. It was also found that rats given an injection of naloxone showed an increase in motor activity and occasionally exhibited behavioral seizures. Subcutaneous injection of 100 mg/kg/day in rats for 3 weeks produced only transient salivation and partial ptosis following injection. Administration of naloxone to rats from day 17 of pregnancy significantly increased neonatal death. Body weight increase was slightly retarded by administration of naloxone. Naloxone was weakly positive in the Ames mutagenicity test and was negative in the in vitro Chinese hamster V79 cell HGPRT mutagenicity assay and the in vivo rat bone marrow chromosome aberration study. Interactions Large doses of naloxone are required to antagonize buprenorphine since the latter has a long duration of action due to its slow rate of binding and subsequent slow dissociation from the opioid receptor. Buprenorphine antagonism is characterized by a gradual onset of the reversal effects and a decreased duration of action of the normally prolonged respiratory depression. The barbiturate methohexital appears to block the acute onset of withdrawal symptoms induced by naloxone in opiate addicts. The combined admin of flunitrazepam and naloxone to volunteers incr resp frequency and resp min vol but did not alter endexpired pCO2 pressure, resp inhalation vol, or alveolar ventilation when compared with naloxone treatment alone. Non-Human Toxicity Values LD50 Mouse iv 90 mg/kg /Naloxone hydrochloride/ LD50 Rat iv 107 mg/kg /Naloxone hydrochloride/ LD50 Rat ip 239 mg/kg /Naloxone hydrochloride/ LD50 Rat sc 500 mg/kg /Naloxone hydrochloride/ LD50 Mouse sc 286 mg/kg /Naloxone hydrochloride/ |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Narcotic Antagonists /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Naloxone is included in the database. Naloxone Hydrochloride Injection, USP is indicated for the complete or partial reversal of opioid depression, including respiratory depression, induced by natural and synthetic opioids, including propoxyphene, methadone, and certain mixed agonist-antagonist analgesics: nalbuphine, pentazocine, butorphanol, and cyclazocine. Naloxone Hydrochloride Injection, USP is also indicated for diagnosis of suspected or known acute opioid overdosage. /Included in US product label/ Naloxone Hydrochloride Injection, USP may be useful as an adjunctive agent to increase blood pressure in the management of septic shock. /Included in US product label/ For more Therapeutic Uses (Complete) data for NALOXONE (14 total), please visit the HSDB record page. Drug Warnings Nausea and vomiting have been reported rarely in postoperative patients who were receiving a parenteral dose of naloxone hydrochloride greater than that usually recommended; however, a causal relationship has not been established. Tremor and hyperventilation associated with an abrupt return to consciousness has occurred in some patients receiving naloxone for opiate overdosage. Although a causal relationship to the drug has not been established, severe cardiopulmonary effects (eg, hypotension, hypertension, ventricular tachycardia and fibrillation, dyspnea, pulmonary edema, cardiac arrest) resulting in death, coma, and encephalopathy have been reported in patients following postoperative administration of naloxone hydrochloride. Adverse cardiopulmonary effects have occurred most frequently in postoperative patients with preexisting cardiovascular disease or in those receiving other drugs that produce similar adverse cardiovascular effects. Seizures have occurred rarely following administration of naloxone hydrochloride; however, a causal relationship to the drug has not been established. When high oral doses of naloxone have been used in the treatment of opiate addiction, some patients have experienced mental depression, apathy, inability to concentrate, sleepiness, irritability, anorexia, nausea, and vomiting. These adverse effects usually occurred in the first few days of treatment and abated rapidly with continued therapy or dosage reduction. One case of erythema multiforme cleared promptly after naloxone was discontinued. For more Drug Warnings (Complete) data for NALOXONE (23 total), please visit the HSDB record page. Pharmacodynamics Naloxone is an opioid receptor antagonist indicated in the reversal of opioid overdoses. Naloxone has a shorter duration of action than opioids and multiple doses may be required. The therapeutic window of naloxone is wide, as it has no effect if a patient has not taken opioids. Patients treated with naloxone may experience opioid withdrawal and a person administering naloxone should be aware that reversal of opioid overdoses may not resolve all the symptoms a patient is experiencing if other drugs are involved. |
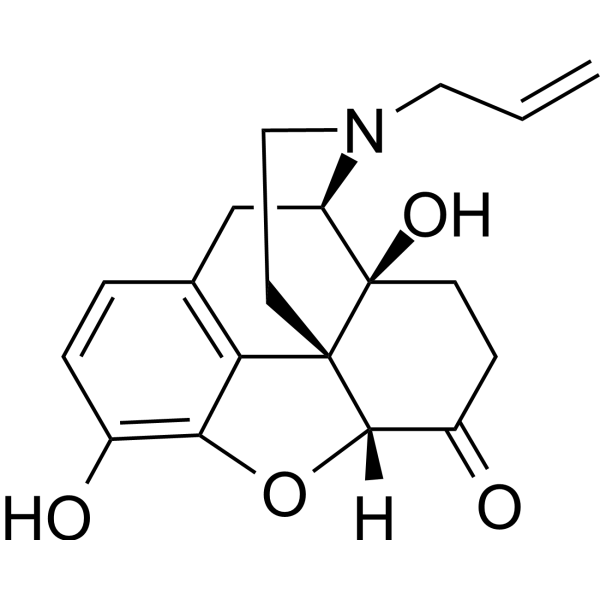
| 分子式 |
C19H21NO4
|
|---|---|
| 分子量 |
327.37
|
| 精确质量 |
327.147
|
| CAS号 |
465-65-6
|
| 相关CAS号 |
Naloxone hydrochloride;357-08-4;Naloxone-d5;1261079-38-2
|
| PubChem CID |
5284596
|
| 外观&性状 |
Crystals from ethyl acetate
|
| 密度 |
1.43 g/cm3
|
| 沸点 |
532.8ºC at 760 mmHg
|
| 熔点 |
184ºC
|
| 闪点 |
276.1ºC
|
| LogP |
1.239
|
| tPSA |
70
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
594
|
| 定义原子立体中心数目 |
4
|
| SMILES |
C=CCN1CC[C@@]23C4=C5C=CC(=C4O[C@H]3C(=O)CC[C@]2([C@H]1C5)O)O
|
| InChi Key |
UZHSEJADLWPNLE-GRGSLBFTSA-N
|
| InChi Code |
InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1
|
| 化学名 |
(4R,4aS,7aR,12bS)-4a,9-dihydroxy-3-prop-2-enyl-2,4,5,6,7a,13-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7-one
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0546 mL | 15.2732 mL | 30.5465 mL | |
| 5 mM | 0.6109 mL | 3.0546 mL | 6.1093 mL | |
| 10 mM | 0.3055 mL | 1.5273 mL | 3.0546 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Efficacy of Spinal Manipulation Therapy or Mindfulness-based Reduction Therapy on Patients With Chronic Low Back Pain
CTID: NCT04744883
PhaseEarly Phase 1 Status: Active, not recruiting
Date: 2024-10-08