| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
- Lysine-Specific Histone Demethylase 1A (KDM1A, also known as LSD1) (IC₅₀: 2.6 nM for recombinant human KDM1A; Ki: 1.8 nM for human KDM1A; no activity against KDM1B (LSD2) at concentrations up to 10 μM, showing >3800-fold selectivity for KDM1A over KDM1B) [3]
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:在 THP-1 (MLL-AF9) 细胞中,ORY-1001 导致 KDM1A 靶基因处时间/剂量依赖性 me2H3K4 积累,并伴随诱导分化标记物。 ORY-1001 还可诱导 THP-1 细胞凋亡,并抑制 MV(4;11) (MLL-AF4) 细胞的增殖和集落形成。激酶测定:ORY-1001 (RG-6016) 是一种口服活性、选择性赖氨酸特异性脱甲基酶 LSD1/KDM1A 抑制剂,IC50<20 nM,对相关 FAD 依赖性氨氧化酶具有高选择性。 ORY-1001 是一种对映体纯的 KDM1A 抑制剂,对相关的 FAD 依赖性氨氧化酶具有高选择性。 ORY-1001 不会抑制非相关组蛋白修饰剂,并且在 CEREP 多样性检测中是干净的。细胞分析:ORY-1001 是一种对映体纯的 KDM1A 抑制剂,对相关的 FAD 依赖性氨氧化酶具有高选择性。 ORY-1001 不会抑制非相关组蛋白修饰剂,并且在 CEREP 多样性检测中是干净的。用 ORY-1001 处理 THP-1 细胞,导致 KDM1A 靶基因处时间/剂量依赖性 me2H3K4 积累,并同时诱导分化标记物。
1. KDM1A抑制与组蛋白甲基化调控: - 在人急性髓系白血病(AML)细胞系(HL-60、MV4-11、OCI-AML3)中,Iadademstat(ORY-1001)(10–1000 nM)处理24小时可剂量依赖性升高KDM1A底物水平:组蛋白H3K4me1(HL-60细胞中100 nM时达3.5±0.4倍)和H3K4me2(HL-60细胞中100 nM时达2.8±0.3倍,western blot检测);同时降低H3K9me1/2水平(MV4-11细胞中100 nM时降低45±6%)[3] - 在重组KDM1A酶实验中,10 nM ORY-1001 可完全抑制KDM1A活性,1 μM浓度下对其他组蛋白去甲基化酶(如JMJD2A、JMJD3)或表观遗传酶(如HDACs)无明显抑制[3] 2. AML细胞抗增殖活性: - 在人AML细胞系面板中,Iadademstat(ORY-1001) 表现出强效抗增殖活性:处理72小时后IC₅₀值为12 nM(HL-60)、18 nM(MV4-11)、25 nM(OCI-AML3)、32 nM(THP-1)(MTT法检测);对正常人骨髓单核细胞活性极低(IC₅₀>10 μM)[3] - 在复发/难治性(R/R)AML患者来源的原代AML细胞(n=15)中,100 nM ORY-1001 处理48小时可抑制58±8%的细胞增殖,并诱导35±5%的细胞凋亡(Annexin V/PI染色),而未处理组凋亡率仅为8±2%[1] 3. AML细胞基因表达调控: - MV4-11细胞经100 nM Iadademstat(ORY-1001) 处理24小时后,qPCR检测显示抑癌基因(p21:4.2±0.5倍,p53:2.1±0.3倍)上调,癌基因(MYC:0.4±0.1倍,BCL-2:0.3±0.1倍)下调[3] |
| 体内研究 (In Vivo) |
每日口服剂量 < 0.020 mg/kg 可显着减少啮齿动物异种移植物中的肿瘤生长。体内研究表明 ORY-1001 具有优异的口服生物利用度、靶标暴露和体内活性。
1. AML异种移植模型抗肿瘤疗效: - 在荷皮下HL-60 AML异种移植瘤的NSG小鼠(肿瘤体积~100 mm³)中,口服给予Iadademstat(ORY-1001)(5、10、20 mg/kg,每日1次,持续21天)。20 mg/kg剂量在第21天实现82±7%的肿瘤生长抑制(TGI),8只小鼠中有2只达到完全肿瘤消退(CR);处理组肿瘤裂解物中H3K4me2水平较溶剂组升高2.3±0.3倍[3] - 在荷原位MV4-11 AML异种移植瘤的NSG小鼠(经尾静脉注射1×10⁶细胞建立模型)中,口服ORY-1001(15 mg/kg/天,持续28天)可将中位生存期从溶剂组的21天延长至38天,生存期获益达81%[3] |
| 酶活实验 |
1. 重组人KDM1A活性实验:
- 将重组人KDM1A(181–836位氨基酸,与CoREST复合)与荧光肽底物(H3K4me2肽,序列ARTKQTARK(me2)STGGKAPRKQL)在assay缓冲液(50 mM Tris-HCl pH 8.0、100 mM NaCl、5 mM DTT、0.1 mg/mL BSA)中于37°C孵育。加入Iadademstat(ORY-1001)(0.1–1000 nM),通过添加2-酮戊二酸(终浓度100 μM)和Fe²⁺(终浓度10 μM)启动反应。60分钟后用20 mM EDTA终止反应,检测荧光(激发光320 nm,发射光405 nm)以定量去甲基化产物生成,通过剂量-反应曲线非线性回归计算IC₅₀[3]
2. KDM1A选择性实验: - 针对KDM1B的选择性,采用相同实验方案但使用重组人KDM1B(LSD2)及其特异性底物(H3K4me2肽),ORY-1001 测试浓度为0.1 nM–10 μM,10 μM以下无KDM1B抑制活性。针对其他表观遗传酶(JMJD2A、JMJD3、HDAC1–3),采用商品化酶活性试剂盒检测,1 μM浓度下无抑制活性[3] |
| 细胞实验 |
1. 抗增殖MTT实验:
- 人AML细胞系(HL-60、MV4-11、OCI-AML3)以5×10³细胞/孔接种于96孔板,用含10%胎牛血清的RPMI 1640培养基培养。加入Iadademstat(ORY-1001)(1 nM–10 μM),孵育72小时后每孔加入10 μL MTT试剂(5 mg/mL),继续孵育4小时;用100 μL DMSO终止反应,检测570 nm处吸光度,通过GraphPad Prism软件计算IC₅₀[3]
2. 组蛋白甲基化western blot实验: - HL-60细胞经ORY-1001(10–1000 nM)处理24小时后,用含蛋白酶和磷酸酶抑制剂的RIPA缓冲液裂解,通过核提取试剂盒制备核提取物;取20 μg核蛋白进行12% SDS-PAGE,转移至PVDF膜。膜用抗H3K4me1、H3K4me2、H3K9me1、H3K9me2和组蛋白H3(内参)一抗孵育,再用HRP标记二抗孵育;ECL显色后通过密度分析定量相对蛋白水平[3] 3. 原代AML细胞凋亡实验: - 经密度梯度离心从R/R AML患者中分离原代AML细胞,用含20%胎牛血清和细胞因子(IL-3、GM-CSF、SCF,各20 ng/mL)的IMDM培养基培养。细胞经100 nM Iadademstat(ORY-1001) 处理48小时后,用Annexin V-FITC和碘化丙啶(PI)室温染色15分钟,流式细胞术分析凋亡(Annexin V⁺/PI⁻为早期凋亡,Annexin V⁺/PI⁺为晚期凋亡)[1] |
| 动物实验 |
1. Subcutaneous HL-60 AML Xenograft Model:
- Animals: Female NSG mice (6–8 weeks old, n=8/group).
- Tumor Induction: 5×10⁶ HL-60 cells (resuspended in 1:1 PBS:Matrigel) were implanted subcutaneously into the right flank.
- Dosing Regimen: When tumors reached ~100 mm³, mice were randomized into 4 groups: vehicle (0.5% methylcellulose + 0.2% Tween 80 in water) and Iadademstat (ORY-1001) at 5, 10, 20 mg/kg. Drugs were administered orally once daily for 21 days.
- Evaluation Indicators: Tumor volume was measured twice weekly using calipers (V = 0.5 × length × width²); body weight was recorded weekly. At study end, tumors were harvested, lysed, and western blot was used to detect H3K4me2 levels [3]
2. Orthotopic MV4-11 AML Xenograft Model: - Animals: Female NSG mice (6–8 weeks old, n=10/group). - Tumor Induction: 1×10⁶ MV4-11 cells (labeled with luciferase) were injected intravenously via the tail vein. Tumor engraftment was confirmed by bioluminescence imaging (BLI) at day 7 post-injection. - Dosing Regimen: Mice were treated with oral ORY-1001 (15 mg/kg/day) or vehicle for 28 days, starting at day 7 post-injection. - Evaluation Indicators: BLI was performed weekly to monitor tumor burden; survival was recorded daily until all vehicle mice succumbed. Median survival and survival benefit were calculated using the Kaplan-Meier method [3] |
| 药代性质 (ADME/PK) |
1. Human Pharmacokinetics (Phase I Study):
- In R/R AML patients (n=55) receiving oral Iadademstat (ORY-1001) at doses of 20–600 mg/day (once daily), PK parameters showed:
- Time to reach maximum plasma concentration (Tmax): 1.5–2.5 hours across all doses.
- Maximum plasma concentration (Cmax): 28.3±5.2 ng/mL (20 mg), 105.6±12.8 ng/mL (100 mg), 320.4±35.7 ng/mL (400 mg).
- Area under the plasma concentration-time curve (AUC₀-24h): 85.6±10.3 ng·h/mL (20 mg), 380.2±42.5 ng·h/mL (100 mg), 1120.5±120.8 ng·h/mL (400 mg) (dose-proportional up to 400 mg).
- Terminal half-life (t₁/₂): 4.2±0.5 hours (consistent across doses).
- Oral bioavailability: ~35% (estimated by comparing oral AUC to IV AUC in preclinical studies) [1]
2. Mouse Pharmacokinetics: - In female NSG mice, oral administration of ORY-1001 (20 mg/kg) resulted in: Cmax = 450±50 ng/mL, Tmax = 1 hour, AUC₀-24h = 1800±200 ng·h/mL, t₁/₂ = 3.8±0.4 hours. Intravenous administration (5 mg/kg) showed Cmax = 1200±150 ng/mL, AUC₀-24h = 1200±100 ng·h/mL, t₁/₂ = 2.1±0.3 hours [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
1. Human Clinical Toxicity (Phase I Study):
- In R/R AML patients treated with Iadademstat (ORY-1001) (20–600 mg/day), treatment-related adverse events (TRAEs) were mostly grade 1–2:
- Common TRAEs (incidence >20%): nausea (42%), fatigue (38%), diarrhea (31%), vomiting (28%), and decreased appetite (22%).
- Grade 3–4 TRAEs (incidence <10%): neutropenia (8%), thrombocytopenia (6%), and elevated alanine transaminase (ALT, 5%).
- Dose-limiting toxicity (DLT): observed at 600 mg/day, consisting of grade 4 neutropenia (duration >7 days) in 2 out of 6 patients [1]
- Plasma protein binding: In human plasma, ORY-1001 showed high protein binding (>98%) as measured by equilibrium dialysis [1] 2. Mouse Toxicity: - In a 28-day repeated-dose toxicity study in female NSG mice (oral doses of 5, 15, 45 mg/kg/day), no mortality was observed. At 45 mg/kg/day, mild weight loss (<10%) and transient elevation of serum AST (1.5-fold above normal) were noted, with no histopathological changes in major organs (liver, kidney, bone marrow) [3] |
| 参考文献 | |
| 其他信息 |
Iadademstat is an orally available inhibitor of lysine specific histone demethylase 1 (KDM1A; LSD1), with potential antineoplastic activity. Upon administration, iadademstat binds to and inhibits LSD1, a demethylase that suppresses the expression of target genes by converting the di- and mono-methylated forms of lysine at position 4 of histone H3 (H3K4) to mono- and unmethylated H3K4, respectively. LSD1 inhibition enhances H3K4 methylation and increases the expression of tumor suppressor genes. This may lead to an inhibition of cell growth in LSD1-overexpressing tumor cells. In addition, LSD1 demethylates mono- or di-methylated H3K9, which increases gene expression of tumor promoting genes; inhibition of LSD1 promotes H3K9 methylation and decreases transcription of these genes. LSD1, an enzyme belonging to the flavin adenine dinucleotide (FAD)-dependent amine oxidase family, is overexpressed in certain tumor cells and plays a key role in in the regulation of gene expression, tumor cell growth and survival.
1. Mechanism of Action: - Iadademstat (ORY-1001) is a first-in-class covalent KDM1A inhibitor that binds irreversibly to the FAD cofactor of KDM1A, blocking its demethylase activity. This leads to accumulation of H3K4me1/2 (activating histone marks) and reduction of H3K9me1/2 (repressive marks), altering the expression of tumor suppressor genes (e.g., p21) and oncogenes (e.g., MYC), thereby inhibiting AML cell proliferation and inducing apoptosis [3] 2. Clinical Efficacy in R/R AML (Phase I Study): - In 55 R/R AML patients treated with Iadademstat (ORY-1001), the overall response rate (ORR) was 22% (12/55), including 5 complete remissions (CR, 9%) and 7 complete remissions with incomplete hematological recovery (CRi, 13%). The median duration of response (DOR) was 5.8 months (range: 2.1–12.3 months) [1] 3. Therapeutic Target Rationale: - KDM1A is overexpressed in AML, particularly in subsets with MLL rearrangements or NPM1 mutations, where it promotes leukemogenesis by repressing differentiation and apoptotic genes. ORY-1001 targets this dependency, making it a promising agent for R/R AML [2][3] |
| 分子式 |
C15H22N2
|
|---|---|
| 分子量 |
230.348583698273
|
| 精确质量 |
230.178
|
| 元素分析 |
C, 78.21; H, 9.63; N, 12.16
|
| CAS号 |
1431304-21-0
|
| 相关CAS号 |
1431303-72-8 (2HCl);1431304-21-0;1431303-71-7 (xHCl);1431326-61-2 (2HCl);
|
| PubChem CID |
71543365
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
2.1
|
| tPSA |
38
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
17
|
| 分子复杂度/Complexity |
239
|
| 定义原子立体中心数目 |
2
|
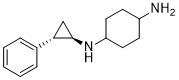
| SMILES |
C1CC(CCC1N)N[C@@H]2C[C@H]2C3=CC=CC=C3
|
| InChi Key |
ALHBJBCQLJZYON-PFSRBDOWSA-N
|
| InChi Code |
InChI=1S/C15H22N2/c16-12-6-8-13(9-7-12)17-15-10-14(15)11-4-2-1-3-5-11/h1-5,12-15,17H,6-10,16H2/t12?,13?,14-,15+/m0/s1
|
| 化学名 |
4-N-[(1R,2S)-2-phenylcyclopropyl]cyclohexane-1,4-diamine
|
| 别名 |
Iadademstat; ORY-1001; ORY-1001 free base; 1431304-21-0; RG-6016; Iadademstat [INN]; CHEMBL3781751; CHEMBL5028924;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.3412 mL | 21.7061 mL | 43.4122 mL | |
| 5 mM | 0.8682 mL | 4.3412 mL | 8.6824 mL | |
| 10 mM | 0.4341 mL | 2.1706 mL | 4.3412 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。