| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
sst1 ( pKi = 8.2 ); sst2 ( pKi = 9.0 ); sst3 ( pKi = 9.1 ); sst4 ( pKi < 7.0 ); sst5 ( pKi = 9.9 )
|
|---|---|
| 体外研究 (In Vitro) |
Pasireotide 对人类生长抑素受体(亚型 sst1/2/3/4/5,pKi 分别为 8.2/9.0/9.1/<7.0/9.9)表现出独特的高亲和力结合[1]。 Pasireotide 可有效抑制大鼠垂体细胞原代培养物中生长激素释放激素 (GHRH) 诱导的生长激素 (GH) 释放,IC50 为 0.4 nM[1]。
本研究中采用的策略得到了回报,与2相比,帕西瑞肽/25具有明显的优势。体外药理学研究清楚地表明,Pasireotide/25有效地抑制了大鼠垂体细胞原代培养中生长激素释放激素(GHRH)诱导的生长激素(GH)释放,IC50为0.4±0.1 nmol/L(n=5)[1]。 Pasireotide是一种生长抑素类似物,与生长抑素受体亚型sst1,2,3和sst5具有高结合亲和力,如使用表达人重组生长抑素受体的CHO-K1细胞的竞争性结合研究所示(表2)(Bruns等人,2002,Schmid和Schoeffter,2004)。在表达人重组sst受体的CCL39细胞中,帕瑞肽和生长抑素(SRIF-14)以大致相同的功效和效力抑制毛喉素刺激的cAMP积累。与奥曲肽相比,帕瑞肽对sst1、sst3和sst5的功能活性(基于EC50值)分别高出30倍、11倍和158倍,但对sst2的功能活性低7倍(Schmid和Schoeffter,2004)。 基于帕瑞肽和奥曲肽的结合亲和力和功能活性的差异,可以推测,在表达sst2受体亚型以外的sst受体的细胞和组织中,帕瑞肽对激素分泌的抑制作用将比奥曲肽更强[3]。 |
| 体内研究 (In Vivo) |
帕瑞肽/Pasireotide(160 mg/kg/口;皮下注射 4 个月)可显着降低 Pdx1-Cre 中的血清胰岛素、升高血糖、减小肿瘤大小并增加细胞凋亡[2]。帕瑞肽(2-50 μg/kg;每天皮下注射两次,持续 42 天)通过 SSTR2 受体在免疫介导的关节炎小鼠模型中发挥抗伤害和抗炎作用[4]。动物模型:12月龄条件性Men1基因敲除胰岛素瘤小鼠[2] 剂量:160 mg/kg/口 给药方式:每月皮下注射,持续4个月 结果:血清胰岛素从1.060 μg/L降低至0.3653 μg/L,并升高血糖从 4.246 mM 降至 7.122 mM。显着减小肿瘤大小并增加细胞凋亡。
在体内,Pasireotide/25也能有效抑制大鼠的GH分泌。注射25后1小时和6小时测定的ED50值表明其在体内的作用持续时间很长。在大鼠中,25显著降低了IGF-1血浆水平,与治疗7天后2引起的效果相比,疗效显著增强。此外,在大鼠、狗和恒河猴中,25在SMS 201-995中观察到的情况下,长时间有效且剂量依赖性地降低IGF-1水平,而没有脱敏(2)。1. 背景:Pasireotide(SOM230)是一种长效生长抑素类似物(LAR),可提高生长抑素受体的激动剂活性。我们使用MEN1转基因小鼠模型测试了SOM230对胰岛素分泌、血糖浓度、肿瘤生长和存活的影响。 方法:对8只12个月龄患有胰岛素瘤的条件性Men1基因敲除小鼠进行评估。治疗组(n=4)和对照组(n=4)每月接受SOM230或PBS的皮下注射。分别通过酶联免疫吸附试验和酶比色法测定血清胰岛素和葡萄糖水平。通过microPET/CT扫描和组织学分析确定肿瘤活性、生长和凋亡。 结果:在第7天,治疗组的血清胰岛素水平从1.06±0.28μg/L降至0.37±0.17μg/L(P=0.0128),血糖从4.2±0.45 mmol/L显著升高至7.12±1.06 mmol/L(P=0.0075),但对照组没有变化。治疗组肿瘤大小(2098±388μm2)小于对照组(7067±955μm2);P=.0024)。此外,与对照组(0.29±0.103%;P=0.002)相比,治疗组的细胞凋亡增加(6.9±1.23%)。 结论:Pasireotide/SOM230在我们的MEN1胰岛素瘤模型中显示出抗分泌、抗增殖和促凋亡活性。有必要进一步研究SOM230对患有MEN1突变的PNET患者的影响。[2] 目的:临床和临床前证据表明,生长抑素具有强效的抗炎和镇痛作用。然而,尚不清楚5种生长抑素受体亚型(SSTRs 1-5)中的哪一种参与了这些作用。本研究旨在评估稳定的生长抑素类似物奥曲肽和Pasireotide(SOM230)在抗原诱导性关节炎(AIA)小鼠模型中的作用。 方法:在SSTR2缺陷小鼠(SSTR2(-/-))及其野生型同窝小鼠(SSTR2+/-)中进行研究。通过免疫组织化学检测背根神经节中SSTR1、SSTR2A、SSTR3和SSTR5的表达。 结果:未经治疗的SSTR2(-/-)AIA小鼠表现出与SSTR2(+/-+)小鼠相似的关节肿胀和机械性痛觉过敏。在野生型小鼠中,奥曲肽和帕瑞肽均显著减轻了膝关节肿胀和关节炎的组织病理学表现,其程度与地塞米松相当。在SSTR2(-/-)小鼠中,奥曲肽和帕瑞肽的抗炎作用被完全消除。长期服用帕西瑞肽也能抑制关节肿胀,并在AIA发作反应中保护关节免受破坏。此外,奥曲肽和帕瑞肽均能减轻炎症性痛觉过敏。奥曲肽在SSTR2(-/-)小鼠中的镇痛作用被消除,但帕瑞肽的镇痛作用得以保留。在幼稚野生型小鼠的背根神经节中,在中小直径神经元的一个子集中只检测到SSTR1和SSTR2A,而没有检测到SSTR3或SSTR5。 结论:我们的研究结果表明,奥曲肽和Pasireotide的镇痛和抗炎作用在很大程度上是通过SSTR2受体介导的。此外,我们发现SSTR1受体是生长抑素介导的炎症性疼痛外周镇痛的新药理学靶点[4]。 |
| 酶活实验 |
药理学特征。放射性配体结合分析。[1]
如前所述进行放射性配体结合分析。简而言之,在存在或不存在不同浓度的SRIF受体配体的情况下,将表达相应人SRIF受体亚型的CHO和COS细胞的膜与SRIF受体配位体Tyr11[125I]-SRIF一起孵育。1小时后,通过Whatman GF/C过滤器快速过滤停止孵育。分析抑制曲线,并计算IC50值。 |
| 细胞实验 |
细胞凋亡分析[2]
通过末端脱氧核苷酸转移酶dUTP缺口末端标记(TUNEL)法测量对照组和治疗组小鼠内分泌肿瘤组织中的凋亡状态。为了定量凋亡,根据制造商的要求,使用原位细胞死亡检测试剂盒对石蜡包埋切片进行TUNEL检测。将组织切片脱蜡,用蛋白酶K(10μg/ml)处理20分钟。然后用PBS洗涤切片两次,在37°C下用TUNEL反应混合物(标记物加酶溶液)标记和染色60分钟,并在黑暗中用PBS洗涤两次。将载玻片安装在含有DAPI的Vectashield安装介质中。在荧光显微镜下计数凋亡的荧光细胞,并将数量表示为总细胞的百分比±标准差(SD)。还进行了未经酶处理的阴性对照和经DNase I处理的阳性对照。 SSTR检测[2] 在我们的小鼠模型中测量了胰腺内分泌肿瘤组织中sstr1-5的检测。使用兔或山羊抗sstr1、sstr2、sstr3、sstr4和sstr5抗体(Abs)对切片上的sstr1-5进行免疫荧光(IF)染色,并分别在4°C下以1:50的稀释度孵育过夜。猪抗胰岛素抗体也用于鉴定β细胞。然后用PBS洗涤切片,并分别在黑暗中用1:200稀释的抗兔或抗山羊Alexa Fluor 488和抗猪Alexa Fluor 647二抗孵育45分钟。还进行了不含一抗的阴性对照。 |
| 动物实验 |
12 month-old conditional Men1 knockout mice with insulinoma
160 mg/kg/mouth S.c. every month for 4 months SOM230/Pasireotide and PBS Administration [2] Mice were anesthetized using halothane and then shaved on their flank for subcutaneous injection of either phosphate buffered saline (PBS) buffer or Pasireotide/SOM230 at a concentration of 160mg/Kg/month (64mg/ml) every month for 4 months. Treatment protocol and drugs. [4] Mice were randomly allocated to the following groups (8–10 animals per experimental condition): 0.9% saline; 2, 20, or 50 μg/kg of octreotide; or 2, 20, or 50 μg/kg of Pasireotide. These doses have been shown to elicit long-lasting therapeutic effects on pituitary hormone secretion in rodents and humans. Octreotide and Pasireotide were a kind gift from Novartis and were administered subcutaneously in a volume of 0.1 ml/kg of body weight. Treatment was started 12 hours before the induction of AIA and was continued for 3, 21, or 42 days, with administration every 12 hours for the indicated time periods. Flare reactions were provoked by injecting the right knee joint cavity with 100 μg of mBSA dissolved in 20 μl of PBS on days 21 and 35 of AIA. An additional group received 0.6 mg/kg of dexamethasone palmitate by intraperitoneal injection. Dexamethasone treatment was carried out for 5 days, followed by a 2-day pause starting 12 hours before AIA induction. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The peak plasma concentration of pasireotide occurs in 0.25-0.5 hours. After administration of single and multiple doses, there is dose-proportionoal increases in Cmax and AUC. Pasireotide is eliminated mostly by hepatic clearance (biliary excretion)(about 48%) with some minor renal clearance (about 7.63%). Pasireotide is widely distributed and has a volume of distribution of >100L. The clearance in healthy patient is ~7.6 L/h and in Cushing’s disease patients is ~3.8 L/h. Metabolism / Metabolites Metabolism is minimal. Biological Half-Life The half-life is 12 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Mild, transient, asymptomatic elevations in serum aminotransferase levels occur in up to 29% of patients receiving pasireotide LAR, but elevations above 5 times the upper limit of normal are rare ( Pasireotide causes inhibition of gall bladder contractility and a decrease in bile secretion, and long term therapy is associated with a high rate of cholesterol gallstone formation. In prospective studies, between 20% and 30% of patients with acromegaly treated with maintenance pasireotide for one to two years developed gallstones detected by ultrasonography and a proportion developed symptomatic cholelithiasis requiring hospitalization and cholecystectomy. Even after cholecystectomy, cholesterol stones may form in the common bile duct and intrahepatic ducts during somatostatin analogue therapy which can cause symptoms and liver test abnormalities. Therapy with ursodiol does not appear to prevent gallstone formation related to somatostatin analogue therapy, although it may help. Likelihood score: E* (unproven but suspected rare cause of clinically apparent hepatobiliary injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation The excretion of pasireotide into breastmilk has not been studied. However, because it has a high molecular weight of 1047 daltons it is likely to be poorly excreted into breastmilk and it is a peptide that is likely digested in the infant's gastrointestinal tract. It is unlikely to reach the clinically important levels in infant serum. However, the manufacturer states that nursing mothers should not use pasireotide. An alternate drug is preferred. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Plasma protein binding is 88%. |
| 参考文献 |
|
| 其他信息 |
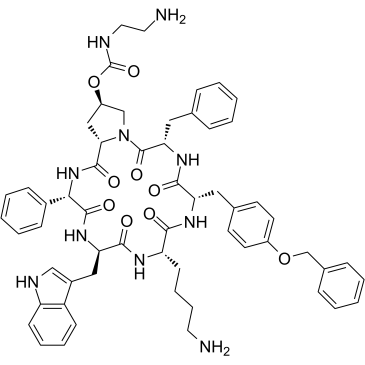
Pasireotide is a six-membered homodetic cyclic peptide composed from L-phenylglycyl, D-tryptophyl, L-lysyl, O-benzyl-L-tyrosyl, L-phenylalanyl and modified L-hydroxyproline residues joined in sequence. A somatostatin analogue with pharmacologic properties mimicking those of the natural hormone somatostatin; used (as its diaspartate salt) for treatment of Cushing's disease. It has a role as an antineoplastic agent. It is a homodetic cyclic peptide and a peptide hormone. It is a conjugate base of a pasireotide(2+).
Pasireotide is a synthetic long-acting cyclic hexapeptide with somatostatin-like activity. It is marketed as a diaspartate salt called Signifor, which is used in the treatment of Cushing's disease. Pasireotide is a Somatostatin Analog. The mechanism of action of pasireotide is as a Somatostatin Receptor Agonist. Pasireotide is a synthetic polypeptide analogue of somatostatin that resembles the native hormone in its ability to suppress levels and activity of growth hormone, insulin, glucagon and many other gastrointestinal peptides. Because its half-life is longer than somatostatin, pasireotide can be used clinically to treat neuroendocrine pituitary tumors that secrete excessive amounts of growth hormone causing acromegaly, or adrenocorticotropic hormone (ACTH) causing Cushing disease. Pasireotide has many side effects including suppression of gall bladder contractility and bile production, and maintenance therapy can cause cholelithiasis and accompanying elevations in serum enzymes and bilirubin. Pasireotide is a synthetic long-acting cyclic peptide with somatostatin-like activity. Pasireotide activates a broad spectrum of somatostatin receptors, exhibiting a much higher binding affinity for somatostatin receptors 1, 3, and 5 than octreotide in vitro, as well as a comparable binding affinity for somatostatin receptor 2. This agent is more potent than somatostatin in inhibiting the release of human growth hormone (HGH), glucagon, and insulin. See also: Pasireotide Diaspartate (is active moiety of); Pasireotide Pamoate (is active moiety of). Drug Indication For the treatment of Cushing’s disease, specifically for those patients whom pituitary surgery has not been curative or is not an option. FDA Label Signifor is indicated for the treatment of adult patients with Cushing's disease for whom surgery is not an option or for whom surgery has failed. Signifor is indicated for the treatment of adult patients with acromegaly for whom surgery is not an option or has not been curative and who are inadequately controlled on treatment with another somatostatin analogue. Treatment of acromegaly and pituitary gigantism Overproduction of pituitary ACTH, Pituitary dependant Cushing's disease, Pituitary dependant hyperadrenocorticism Mechanism of Action Pasireotide activates a broad spectrum of somatostatin receptors, exhbiting a much higher binding affinity for somatostatin receptors 1, 3, and 5 than octreotide in vitro, as well as a comparable binding affinity for somatostatin receptor 2. The binding and activation of the somatostatin receptors causes inhibition of ACTH secretion and results in reduced cortisol secretion in Cushing's disease patients. Also this agent is more potent than somatostatin in inhibiting the release of human growth hormone (HGH), glucagon, and insulin. Pharmacodynamics Signifor® is an analogue of somatostatin that promotes reduced levels of cortisol secretion in Cushing's disease patients. A rational drug design approach, capitalizing on structure-activity relationships and involving transposition of functional groups from somatotropin release inhibitory factor (SRIF) into a reduced size cyclohexapeptide template, has led to the discovery of SOM230 (25), a novel, stable cyclohexapeptide somatostatin mimic that exhibits unique high-affinity binding to human somatostatin receptors (subtypes sst1-sst5). SOM230 has potent, long-lasting inhibitory effects on growth hormone and insulin-like growth factor-1 release and is a promising development candidate currently under evaluation in phase I clinical trials. [1] Pasireotide (SOM230) is a multi-receptor ligand somatostatin analogue with high binding affinity for somatostatin receptor subtypes sst(1,2,3) and sst(5). Pasireotide potently suppresses GH, IGF-I and ACTH secretion, indicating potential efficacy in acromegaly and Cushing's disease. The prolonged inhibition of hormone secretion by pasireotide in animal models and expression of multiple sst receptors in carcinoid tumors suggests that pasireotide may have clinical advantages over octreotide in patients with carcinoid tumors. Direct and indirect antitumor activity has been observed in vitro with pasireotide, including sst receptor-mediated apoptosis and antiangiogenesis, suggesting a possible role for pasireotide in antineoplastic therapy. In summary, preclinical evidence, as well as preliminary results from clinical studies suggests that pasireotide is a promising new treatment for patients with symptoms of metastatic carcinoid tumors refractory or resistant to octreotide, de novo or persistent acromegaly, and that pasireotide has the potential to be the first directed medical therapy for Cushing's disease.[3] In summary, this study is the first of its kind to demonstrate the antisecretory, antiproliferative and proapoptotic actions of the novel long acting release somatostatin analogue SOM230 in a mouse model of insulinomas with improved overall survival. The enhanced spectrum of activity of SOM230 is a result of its enhanced activity at 4 of the 5 sstr subtypes: sstr5, sstr2, sstr3, and sstr1. This is of particular clinical importance in unresectable or metastatic insulinomas that are relatively unresponsive to traditional therapy with octreotide and/or diazoxide. Treatment with SOM230 may facilitate symptomatic relief and result in tumor regression. We believe that this novel strategy of targeted therapy with SOM230 will be of benefit to patients with PNETs. While these data strongly support the effects of pasireotide in this model, we acknowledge that further studies with larger sample sizes are warranted to advance this novel approach toward clinical trials. [2] We also studied the progression of histopathologic changes of chronic inflammation after prolonged administration of pasireotide. These changes become particularly evident upon repeated injections of the antigen into the joint. The present data clearly show that in this model of immune-mediated arthritis, not only the acute inflammatory reaction, but also the chronic inflammatory/destructive process, can be dampened by SSTR agonists. Due to their antiinflammatory actions (equivalent to dexamethasone) and their antinociceptive actions, SSTR agonists are of interest for clinical therapy. In fact, in a pilot study in RA patients, significant clinical improvement was noted after treatment with octreotide. The present observations suggest that pasireotide and octreotide would exhibit similar antiinflammatory effects; however, pasireotide would be expected to provide better pain control than octreotide in RA. As an important aspect, treatment with somatostatin analogs is considered to be relatively safe and well tolerated. Moreover, for prolonged use, long-acting versions of both octreotide and pasireotide are available; these need to be administered only once a month, thus eliminating the need for daily subcutaneous injections. In conclusion, we provide evidence of potent antiinflammatory and antinociceptive effects of the somatostatin receptor agonists octreotide and pasireotide. We identify SSTR2 as an important target involved in the antiinflammatory effects of somatostatin. Both SSTR2 and SSTR1 mediate antinociception. Concerning the clinical use of SSTR agonists, it should be considered that pan-SSTR agonists may be superior to selective SSTR2 agonists.[4] |
| 分子式 |
C58H66N10O9
|
|---|---|
| 分子量 |
1047.2062535286
|
| 精确质量 |
1046.501
|
| 元素分析 |
C, 66.52; H, 6.35; N, 13.38; O, 13.75
|
| CAS号 |
396091-73-9
|
| 相关CAS号 |
Pasireotide acetate; 396091-76-2; Pasireotide ditrifluoroacetate; Pasireotide L-aspartate salt; 396091-77-3; Pasireotide pamoate; 396091-79-5; Pasireotide (diaspartate); 1421446-02-7; 820232-50-6 (diaspartate)
|
| PubChem CID |
9941444
|
| 序列 |
Cyclo[{4-(NH2-C2H4-NH-CO-O-)Pro}-Phg-{D-Trp}-Lys-{Tyr(4-Bzl)}-Phe]
|
| 短序列 |
Cyclo[{4-(NH2-C2H4-NH-CO-O-)Pro}-Phg-{D-Trp}-K-{Tyr(4-Bzl)}-F]
|
| 外观&性状 |
White to off-white Solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
1351.4±65.0 °C at 760 mmHg
|
| 闪点 |
771.1±34.3 °C
|
| 蒸汽压 |
0.0±0.3 mmHg at 25°C
|
| 折射率 |
1.680
|
| LogP |
2.79
|
| tPSA |
281.2
|
| 氢键供体(HBD)数目 |
9
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
18
|
| 重原子数目 |
77
|
| 分子复杂度/Complexity |
1940
|
| 定义原子立体中心数目 |
7
|
| SMILES |
NCCCC[C@@H](C(N[C@H]1CC2=CC=C(C=C2)OCC3=CC=CC=C3)=O)NC([C@H](NC([C@H](C4=CC=CC=C4)NC([C@H]5N(C([C@H](CC6=CC=CC=C6)NC1=O)=O)C[C@H](OC(NCCN)=O)C5)=O)=O)CC7=CNC8=C7C=CC=C8)=O
|
| InChi Key |
VMZMNAABQBOLAK-DBILLSOUSA-N
|
| InChi Code |
InChI=1S/C58H66N10O9/c59-27-13-12-22-46-52(69)64-47(30-38-23-25-42(26-24-38)76-36-39-16-6-2-7-17-39)53(70)66-49(31-37-14-4-1-5-15-37)57(74)68-35-43(77-58(75)61-29-28-60)33-50(68)55(72)67-51(40-18-8-3-9-19-40)56(73)65-48(54(71)63-46)32-41-34-62-45-21-11-10-20-44(41)45/h1-11,14-21,23-26,34,43,46-51,62H,12-13,22,27-33,35-36,59-60H2,(H,61,75)(H,63,71)(H,64,69)(H,65,73)(H,66,70)(H,67,72)/t43-,46+,47+,48-,49+,50+,51+/m1/s1
|
| 化学名 |
[(3S,6S,9S,12R,15S,18S,20R)-9-(4-aminobutyl)-3-benzyl-12-(1H-indol-3-ylmethyl)-2,5,8,11,14,17-hexaoxo-15-phenyl-6-[(4-phenylmethoxyphenyl)methyl]-1,4,7,10,13,16-hexazabicyclo[16.3.0]henicosan-20-yl] N-(2-aminoethyl)carbamate
|
| 别名 |
Pasireotide free base; SOM230; Pasireotide; 396091-73-9; pasireotida; pasireotidum; CHEBI:72312; UNII-98H1T17066; 98H1T17066; Cyclo((4R)-4-(2-aminoethylcarbamoyloxy)-L-prolyl-L-phenylglycyl-D-tryptophyl-L-lysyl-4-O-benzyl-L-tyrosyl-L-phenylalanyl-); SOM-230; SOM 230; trade name: Signifor; Signifor LAR
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~100 mg/mL (~95.5 mM)
Ethanol: ~100 mg/mL |
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.9549 mL | 4.7746 mL | 9.5492 mL | |
| 5 mM | 0.1910 mL | 0.9549 mL | 1.9098 mL | |
| 10 mM | 0.0955 mL | 0.4775 mL | 0.9549 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Reduction by Pasireotide of the Effluent Volume in High-output Enterostomy in Patients Refractory to Usual Medical Treatment
CTID: NCT02713776
Phase: Phase 2 Status: Completed
Date: 2022-04-04
|