| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
EZH2 (Ki = 2.5 nM); EZH2 (IC50s = 11 nM, 16 nM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:EPZ-6438 浓度依赖性地降低野生型或 SMARCB1 突变细胞中的整体 H3K27Me3 水平,并在 SMARCB1 缺失的 MRT 细胞系中诱导强烈的抗增殖作用,IC50 范围为 32 nM 至 1000 nM。 EPZ-6438 诱导神经元分化和细胞周期抑制的基因表达,同时抑制 Hedgehog 通路基因 MYC 和 EZH2 的表达。在几种 EZH2 突变淋巴瘤细胞系中,泼尼松龙或地塞米松可增强 EPZ-6438 的抗增殖作用。激酶测定:EPZ-6438 在 1X 测定缓冲液(20 mM Bicine [pH 7.6]、0.002% Tween-20、每孔 40 μL 5 nM PRC2(50 μL 中的最终测定浓度为 4 nM)中孵育 30 分钟, 0.005% 牛皮明胶和 0.5 mM DTT)。每孔添加 10 μL 底物混合物,其中包含测定缓冲液 3 H-SAM、未标记的 SAM 和代表组蛋白 H3 残基 21-44 的肽(含有 C 端生物素(附加到 C 端酰胺封端的赖氨酸))以启动反应(两种底物以其各自的 Km 值存在于最终反应混合物中,这种测定形式称为“平衡条件”。针对每种酶指示了底物的最终浓度和底物肽的甲基化状态。反应孵育室温下 90 分钟,每孔加入 10 μL 600 μM 未标记 SAM,然后转移至 384 孔 flashplate,30 分钟后清洗。 细胞测定:对于贴壁细胞系增殖测定,每个细胞系的铺板密度为根据生长曲线(通过 ATP 含量测量)和 7 天时间过程中的密度确定。在化合物处理前一天,将细胞一式三份接种在 96 孔板中(第 0-7 天的时间过程)或6 孔板(用于在第 7 天重新接种以完成剩余的时间过程)。第 0 天,细胞未经处理、DMSO 处理或用 EPZ-6438 处理(从 10 µM 开始并以三倍或四倍稀释度逐渐减少)。使用 Cell Titer Glo 在第 0 天、第 4 天和第 7 天对板进行读数,并在第 4 天补充化合物/培养基。在第 7 天,将六孔板用胰蛋白酶消化、离心并重悬于新鲜培养基中,以便通过以下方法进行计数: Vi-细胞。将每次处理的细胞以原始密度重新接种到 96 孔板中,一式三份。让细胞在平板上粘附过夜,并按第 0 天处理细胞。在第 7、11 和 14 天,使用 Cell Titer Glo 读取平板,并在第 11 天补充化合物/培养基。一式三份的平均值为用于绘制随时间变化的增殖曲线,并计算 IC50 值。对于细胞周期和细胞凋亡,将 G401 和 RD 细胞以每板 1 × 106 个细胞的密度一式两份铺在 15 cm 培养皿中。将细胞与 1 µM 的 EPZ-6438(总共 25 mL)一起孵育 14 天,并在第 4、7 和 11 天将细胞分裂回原始铺板密度。细胞周期分析和 TUNEL 测定使用番石榴流式细胞仪按照制造商的方案进行。
|
||
| 体内研究 (In Vivo) |
在携带 sc G401 异种移植物的 SCID 小鼠中,Tazemetostat (EPZ6438)在给药期间诱导肿瘤停滞,并产生显着的肿瘤生长延迟,而对体重的影响最小。
Tazemetostat (EPZ6438)导致SMARCB1突变体MRT异种移植物完全和持续的退化 在移植了s.c G401异种移植物的SCID小鼠中进行了一项研究,动物口服EPZ-6438 21 d。每组一半的小鼠在第21天被安乐死以收集血液和组织,而其余的动物被额外治疗7天,然后不给药32天。EPZ-6438在所有剂量下都具有良好的耐受性,对体重的影响最小(图S4A)。口服剂量为250或500 mg/kg,每日两次(BID),持续21-28天,几乎消除了快速生长的G401肿瘤(图S4 B和C以及图4A)。停药后32 d未见再生。以125 mg/kg剂量给药的EPZ-6438在给药期间诱导肿瘤停滞,给药后肿瘤生长明显延迟。在第21天给药前5分钟或给药后3小时测量EPZ-6438血浆水平显示出明显的剂量依赖性全身暴露增加(图S4D)。在第21天,从各组小鼠亚群中收获的肿瘤显示出对H3K27Me3的强烈抑制,与抗肿瘤活性相关(在250 mg/kg时达到最大效果;图4 b)。此外,在G401异种移植肿瘤中检测到CD133、PTPRK、DOCK4和GLI1表达的剂量依赖性变化。 |
||
| 酶活实验 |
生化酶测定[2]
重组纯化的人PRC2复合物含有WT EZH2、Y641 EZH2突变体、A677G EZH2或WT EZH1,如前所述1,2。鸡红细胞寡核小体按照前面描述的方法纯化。生物素化组蛋白肽由21世纪生物化学公司合成,高效液相色谱纯化纯度为95%。384孔闪片和Microscint 0闪烁液购自商业公司,96孔Multiscreen HTS滤网结合板购自Millipore公司。3H标记的S-腺苷蛋氨酸(3H- SAM)经市售获得,比活性为80 Ci/mmol。未标记的SAM和S-‐腺苷型同型半胱氨酸(SAH)在商业上获得。闪光板在Biotek ELx- 405中用0.1%的Tween洗涤。384孔闪光板和96孔滤镜结合板在TopCount微孔板读取仪上读取。在Freedom EVO上进行化合物系列稀释,并使用Thermo Scientific Matrix PlateMate将其标记到分析板上。 酶抑制IC50值测定[2] EPZ005687在DMSO中连续稀释3倍,从2.5 mM开始(化合物的最终最高浓度为50 μM, DMSO为2%),绘制10点曲线。在384孔微滴板上检测1 μL抑制剂稀释系列。100%抑制对照为1mm终浓度的产物抑制剂S—‐腺苷型同型半胱氨酸(SAH)。化合物在1X缓冲液(20 mM比辛[pH 7.6], 0.002%吐温20,0.005%牛皮明胶和0.5 mM DTT)中以每孔40 μL 5 nM PRC2 (50 μL最终测定浓度为4 nM)孵育30 min。每孔加入10 μL的底物混合物,包括实验缓冲液3H- SAM,未标记的SAM和代表组蛋白H3残基21 - 44的肽,其中含有C-末端生物素(附加在C-末端酰胺覆盖的赖氨酸上),以启动反应(两种底物都以各自的Km值存在于最终的反应混合物中,实验格式称为“平衡条件”4。底物的最终浓度和底物肽的甲基化状态在补充表7中显示。反应在室温下孵育90分钟,用每孔10 μL的600 μM未标记的SAM淬灭,然后转移到384孔的Flashplate上,30分钟后清洗。 EPZ005687——底物竞争[2] 384孔中50 μL总容积的SAM竞赛实验条件与IC50实验相似,但有以下不同。EPZ005687在DMSO(终浓度2%)中连续稀释2倍,得到10点曲线。在0.012 ~ 15 μM范围内对SAM进行滴定。为了监测反应,在每个反应中使用放射性示踪剂3H—‐SAM,相当于高达250 nM(占总SAM浓度)。当SAM浓度小于250 nM时,反应中只含有3H—‐SAM。每个SAM浓度都有对应的孔,反应中不存在酶作为阴性对照,以减去3H—‐SAM对背景信号的贡献。反应孵育90 min后,以每孔10 μL的600 μM SAM溶液淬灭。寡核小体竞争在与IC50测定中描述的相同的实验缓冲液中以96孔格式进行,只是补充了100 mM KCl。EPZ005687在DMSO(终浓度2%)中连续稀释2倍,得到10点曲线。寡核小体用2倍稀释方案滴定8个点,最高浓度为500 nM,最终酶反应体积为50 μL。反应通过加入SAM和3H-‐SAM的混合物开始,最终浓度分别为450和150 nM。加入10 μL的SAM (600 μM)淬灭反应,并将30 μL的反应加入96孔过滤结合板中。用200 μL 10%三羧酸洗涤3次,再用95%乙醇洗涤1次。将膜风干,加入30 μL Microscint 0,然后在TopCount中读取。 磁下拉EZH2以确定EPZ005687对PRC2复合物内蛋白-蛋白相互作用的影响[2] Anti- FLAG M2磁珠的50%悬浮液在1X实验缓冲液中洗涤3次,缓冲液由20 mM比辛(pH = 7.6)和0.002% Tween20组成。含有FLAG标记EED的PRC2复合物(500 nM)与10 μM EPZ005687在1X实验缓冲液中孵育。添加的化合物对DMSO的贡献为1%,因此还包括1% DMSO的车辆对照。然后将孵育液加入50 μL洗涤珠中,在96孔聚丙烯微孔板的孔中重悬于50 μL 1X实验缓冲液中,室温孵育1小时。用微孔板磁铁拉下微珠,取50 μL上清。用100 μL 1X缓冲液洗涤3次,用50 μL 1X缓冲液重悬。然后将上清液和微球与等体积的2X SDS-‐PAGE凝胶上样缓冲液混合,煮沸10分钟。样品在8% Tris -- -甘氨酸SDS -- - PAGE凝胶上在125V下运行90分钟,然后用GelCode Blue染色。 Yonetani—theorll分析法测定SAH与EPZ005687的互斥结合[2] SAH和EPZ005687被连续稀释,并在384孔的微孔板上以网格模式进行标记,这样就可以获得SAH和EPZ005687的所有浓度组合。为此,40 μL含有PRC2 (4 nM)和生物素的混合物在1X实验缓冲液中(20 mM比辛[pH 7.6], 0.002%吐温20,0.005%牛皮明胶和0.5 mM DTT)。每孔加入10 μL底物混合物,包括实验缓冲液3H- SAM (150 nM)、未标记的SAM (1800 nM)和代表组蛋白H3残基21 - 44(含C-末端生物素(附加在C-末端酰胺覆盖的赖氨酸上)的肽段(185 nM),以启动反应(两种底物都以各自的Km值存在于最终的反应混合物中,实验格式称为“平衡条件”4。反应在室温下孵育90分钟,用每孔10 μL的600 μM未标记的SAM淬灭,然后转移到384孔的Flashplate上,30分钟后清洗。在几种不同浓度的EPZ005687下,反应速度的反比作为SAH浓度的函数绘制,得到Yonetani—‐theorll图。 EPZ005687对77个人体离子通道和gpcr的选择性研究[2] EPZ005687在Cerep提供的标准面板上进行了放射性标记配体的位移测试,已知这些配体可以结合77个人体离子通道和gpcr。EPZ005687在10 μM的浓度下进行了重复测试,并将受体的特异性配体结合定义为在过量未标记配体存在的情况下确定的总结合和非特异性结合之间的差异。补充表1中的结果表示为特异性结合控制的百分比((测量的特异性控制/特异性控制结合)× 100)。补充表1还显示了参考放射配体的身份及其结合亲和力。 |
||
| 细胞实验 |
体外细胞测定。[1]
对于贴壁细胞系增殖试验[除KYM-1外的所有细胞系,KYM-1按照先前描述的悬浮细胞系进行分析],每个细胞系的镀密度是根据生长曲线(通过ATP含量测量)和密度在7 d时间过程中确定的。在复合处理前一天,将细胞分别镀于96孔板(0-7天)或6孔板(第7天重复,其余时间)。在第0天,细胞分别未经处理、dmso处理或用<强> <强>的Tazemetostat (EPZ6438)处理,从10µM开始,以三倍或四倍的稀释度递减。在第0天、第4天和第7天使用Cell Titer Glo读取培养皿,第4天补充化合物/培养基。第7天,将六孔板胰蛋白酶化,离心,在新鲜培养基中重悬,用Vi-Cell计数。每个处理的细胞按原始密度在96孔板上复制三份。细胞贴壁过夜,细胞处理与第0天一样。在第7、11和14天,使用细胞滴度荧光仪(Cell Titer Glo)读取培养皿,并在第11天补充化合物/培养基。使用三个重复的平均值来绘制随时间过程的增殖情况,并计算IC50值。G401和RD细胞以1 × 106个细胞/板的密度,在15 cm培养皿中一式两份进行细胞周期和凋亡检测。将细胞与的Tazemetostat (EPZ6438)在1µM中孵育,共25 mL,持续14 d,在第4,7和11天将细胞分裂回原始镀密度。使用Guava流式细胞仪进行细胞周期分析和TUNEL测定,遵循制造商的方案。 基因表达分析。[1] G401细胞和RD细胞分别以175,000个/瓶和117,000个/瓶的速度在T-75烧瓶中镀,并允许粘附过夜。第0天,用DMSO或1µM Tazemetostat (EPZ6438)处理细胞。在第2、4和7天收获细胞并成球,第4天补充培养基和化合物。使用剂量为21 d的G401异种移植动物肿瘤组织[对照、125 mg/kg、250 mg/kg(各6只)和500 mg/kg(4只)<强>他泽美他汀(EPZ6438)<强>剂量组]进行基因表达分析。使用RNeasy Mini Kit从细胞颗粒和肿瘤组织中提取总mRNA,并使用High Capacity cDNA逆转录Kit进行逆转录。RT-PCR采用ViiA 7 Real-Time PCR系统(AB),使用TaqMan Fast Advanced Master Mix (AB;4444964)和TaqMan引物/探针组。基因表达归一化为18S (AB);Hs99999901_s1),折线变化采用ΔΔCt方法计算。对体内样品,取各给药组的平均Ct值±SD,并采用ΔΔCt方法计算与载药组比较的倍数变化。 |
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Tazemetostat 800mg twice daily leads to a Cmax of 829ng/mL, with a Tmax of 1-2 hours , and an AUC of 3340ng\*h/mL. Absorption is not significantly affected by a high fat, high calorie meal. Tazemetostat is 33% bioavailable. Tazemetostat is 15% eliminated in urine and 79% eliminated in feces. Tazemetostat has a volume of distribution of 1230L. Tazemetostat has an apparent total clearance of 274L/h. Metabolism / Metabolites Tazemetostat is metabolized by CYP3A4 to an inactive desethyl metabolite and one other inactive metabolite not described. Biological Half-Life Tazemetostat has a terminal elimination half life of 3.1h. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In clinical trials, serum ALT elevations occurred in 14% and AST elevations in 18% of patients on tazemetostat therapy and rose to more than 5 times ULN in 3.5%. Nevertheless, there were no instances of clinically apparent liver injury with symptoms or jaundice in several multicenter open-label trials of tazemetostat. Despite the frequency of adverse events during tazemetostat therapy, discontinuations due to adverse events are uncommon. Clinical experience with tazemetostat, however, is limited and the frequency of de novo serum enzyme elevations during treatment raises the issue of its potential for causing hepatotoxicity. Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury). Protein Binding Tazemetostat is 88% protein bound in plasma. |
||
| 参考文献 |
|
||
| 其他信息 |
Tazemetostat is a methyltransferase inhibitor used to treat metastatic or locally advanced epithelioid sarcoma not eligible for complete resection. Tazemetostat was first named in literature as EPZ-6438. Tazemetaostat was granted FDA approval on 23 January 2020.
Tazemetostat is a Methyltransferase Inhibitor. The mechanism of action of tazemetostat is as a Methyltransferase Inhibitor, and Multidrug and Toxin Extrusion Transporter 1 Inhibitor, and Multidrug and Toxin Extrusion Transporter 2 K Inhibitor. Tazemetostat is a methyltransferase inhibitor and antineoplastic agent used in the therapy of advanced epithelioid sarcoma. Tazemetostat is associated with a moderate rate of transient serum enzyme elevations during therapy, but has not been implicated in cases of clinically apparent acute liver injury with jaundice. Tazemetostat is an orally available, small molecule selective and S-adenosyl methionine (SAM) competitive inhibitor of histone methyl transferase EZH2, with potential antineoplastic activity. Upon oral administration, tazemetostat selectively inhibits the activity of both wild-type and mutated forms of EZH2. Inhibition of EZH2 specifically prevents the methylation of histone H3 lysine 27 (H3K27). This decrease in histone methylation alters gene expression patterns associated with cancer pathways and results in decreased tumor cell proliferation in EZH2 mutated cancer cells. EZH2, which belongs to the class of histone methyltransferases (HMTs), is overexpressed or mutated in a variety of cancer cells and plays a key role in tumor cell proliferation. See also: Tazemetostat Hydrobromide (active moiety of); Tazemetostat hydrochloride (is active moiety of); Tazemetostat dihydrobromide (is active moiety of) ... View More ... Drug Indication Tazemetostat is indicated to treat adult and pediatric patients 16 years and older with metastatic or locally advanced epithelioid sarcoma that is not eligible for complete resection. It is also indicated to treat adult patients with relapsed or refractory follicular lymphoma whose tumors are positive for an IZH2 mutation and who have received at least 2 prior systemic therapies. Additionally, it is indicated in adult patients with relapsed or refractory follicular lymphoma who have no satisfactory alternative treatment options. Mechanism of Action EZH2 is a methyltransferase subunit of the polycomb repressive complex 2 (PRC2) which catalyzes multiple methylations of lysine 27 on histone H3 (H3K27). Trimethylation of this lysine inhibits the transcription of genes associated with cell cycle arrest. PRC2 is antagonized by the switch/sucrose non-fermentable (SWI/SNF) multiprotein complex. Abnormal activation of EZH2 or loss of function mutations in SWI/SNF lead to hyper-trimethylation of H3K27. Hyper-trimethylation of H3K27 leads to cancer cell de-differentiation, a gain of cancer stem cell-like properties. De-differentiation can allow for cancer cell proliferation. Tazemetostat inhibits EZH2, preventing hyper-trimethylation of H3K27 and an uncontrollable cell cycle. Pharmacodynamics Tazemetostat is a methyltransferase inhibitor that prevents hyper-trimethylation of histones and inhibits cancer cell de-differentiation. The duration of action is long as it is given twice daily. Patients should be counselled regarding the risk of secondary malignancies and embryo-fetal toxicity. Inactivation of the switch/sucrose nonfermentable complex component SMARCB1 is extremely prevalent in pediatric malignant rhabdoid tumors (MRTs) or atypical teratoid rhabdoid tumors. This alteration is hypothesized to confer oncogenic dependency on EZH2 in these cancers. We report the discovery of a potent, selective, and orally bioavailable small-molecule inhibitor of EZH2 enzymatic activity, (N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(morpholinomethyl)-[1,1'-biphenyl]-3-carboxamide). The compound induces apoptosis and differentiation specifically in SMARCB1-deleted MRT cells. Treatment of xenograft-bearing mice with (N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(morpholinomethyl)-[1,1'-biphenyl]-3-carboxamide) leads to dose-dependent regression of MRTs with correlative diminution of intratumoral trimethylation levels of lysine 27 on histone H3, and prevention of tumor regrowth after dosing cessation. These data demonstrate the dependency of SMARCB1 mutant MRTs on EZH2 enzymatic activity and portend the utility of EZH2-targeted drugs for the treatment of these genetically defined cancers.[1] EZH2 catalyzes trimethylation of histone H3 lysine 27 (H3K27). Point mutations of EZH2 at Tyr641 and Ala677 occur in subpopulations of non-Hodgkin's lymphoma, where they drive H3K27 hypertrimethylation. Here we report the discovery of EPZ005687, a potent inhibitor of EZH2 (K(i) of 24 nM). EPZ005687 has greater than 500-fold selectivity against 15 other protein methyltransferases and has 50-fold selectivity against the closely related enzyme EZH1. The compound reduces H3K27 methylation in various lymphoma cells; this translates into apoptotic cell killing in heterozygous Tyr641 or Ala677 mutant cells, with minimal effects on the proliferation of wild-type cells. These data suggest that genetic alteration of EZH2 (for example, mutations at Tyr641 or Ala677) results in a critical dependency on enzymatic activity for proliferation (that is, the equivalent of oncogene addiction), thus portending the clinical use of EZH2 inhibitors for cancers in which EZH2 is genetically altered.[2] |
| 分子式 |
C34H45CLN4O4
|
|---|---|
| 分子量 |
609.20
|
| 精确质量 |
608.312
|
| 元素分析 |
C, 67.03; H, 7.45; Cl, 5.82; N, 9.20; O, 10.50
|
| CAS号 |
1467052-84-1
|
| 相关CAS号 |
1467052-84-1 (HCl);1467052-75-0 (HBr);1403254-99-8;
|
| PubChem CID |
91617699
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| tPSA |
83.1
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
43
|
| 分子复杂度/Complexity |
992
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
CJPMOJLLSLWWHI-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C34H44N4O4.ClH/c1-5-38(29-10-14-41-15-11-29)32-20-28(27-8-6-26(7-9-27)22-37-12-16-42-17-13-37)19-30(25(32)4)33(39)35-21-31-23(2)18-24(3)36-34(31)40;/h6-9,18-20,29H,5,10-17,21-22H2,1-4H3,(H,35,39)(H,36,40);1H
|
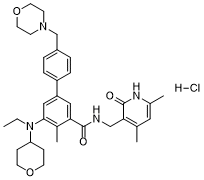
| 化学名 |
N-[(4,6-dimethyl-2-oxo-1H-pyridin-3-yl)methyl]-3-[ethyl(oxan-4-yl)amino]-2-methyl-5-[4-(morpholin-4-ylmethyl)phenyl]benzamide;hydrochloride
|
| 别名 |
Tazemetostat hydrochloride; Tazemetostat monohydrochloride; EPZ-6438 monohydrochloride; 1467052-84-1; UNII-1L6UQ905WM; 1L6UQ905WM; Tazemetostat (hydrochloride); (1,1'-Biphenyl)-3-carboxamide, N-((1,2-dihydro-4,6-dimethyl-2-oxo-3-pyridinyl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(4-morpholinylmethyl)-, hydrochloride (1:1);
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6415 mL | 8.2075 mL | 16.4150 mL | |
| 5 mM | 0.3283 mL | 1.6415 mL | 3.2830 mL | |
| 10 mM | 0.1641 mL | 0.8207 mL | 1.6415 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05228158 | Recruiting | Drug: Tazemetostat | Lymphoma, Follicular | Eisai Co., Ltd. | August 16, 2021 | |
| NCT05934838 | Recruiting | Drug: Tazemetostat Pill | Follicular Lymphoma B-Cell Lymphoma |
Weill Medical College of Cornell University |
October 4, 2023 | Phase 1 |
| NCT05023655 | Recruiting | Drug: Tazemetostat | Solid Tumor ARID1A Gene Mutation |
Prisma Health-Upstate | January 6, 2022 | Phase 2 |
| NCT05994235 | Recruiting | Drug: Mosunetuzumab Drug: Tazemetostat Pill |
Follicular Lymphoma | Weill Medical College of Cornell University |
November 1, 2023 | Phase 2 |