| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
α1-adrenoceptor
α1-Adrenergic Receptor (α1-AR) subtypes (α1A Ki = 3.2 nM; α1B Ki = 4.7 nM; α1D Ki = 5.1 nM) [2] - α1-AR (IC50 = 12 nM for norepinephrine-induced vascular smooth muscle contraction inhibition) [1] |
|---|---|
| 体外研究 (In Vitro) |
体外活性:Terazosin 在 PC-3 和人良性前列腺细胞中诱导细胞毒性,IC50 超过 100 μM。特拉唑嗪还有效抑制培养的人脐静脉内皮细胞中血管内皮生长因子诱导的增殖和管形成(IC50 分别为 9.9 和 6.8 μM)。 细胞测定:为了确定细胞毒性作用的作用方式,本研究中使用了几种鉴定技术。使用末端脱氧核苷酸转移酶脱氧尿苷三磷酸缺口末端标记原位检测凋亡细胞。结果显示,用特拉唑嗪 (100 μM) 处理 PC-3 细胞 12 小时后出现阳性反应。
盐酸特拉唑嗪(Terazosin HCl) 是选择性α1肾上腺素能受体拮抗剂,对所有α1-AR亚型(α1A、α1B、α1D)具有高亲和力,Ki值范围为3.2 nM至5.1 nM[2] - 在分离的大鼠主动脉平滑肌条中,盐酸特拉唑嗪(0.1–100 nM)剂量依赖性抑制去甲肾上腺素诱导的收缩。12 nM(IC50)时收缩幅度降低50%,100 nM时抑制率达82%[1] - 在人前列腺基质细胞(WPMY-1)中,盐酸特拉唑嗪(1–20 μM)剂量依赖性抑制细胞增殖。10 μM处理72小时后,细胞活力降低45%,并诱导G1期细胞周期阻滞(G1期细胞比例从42%升至68%)[3] - 盐酸特拉唑嗪(5 μM)阻断大鼠血管平滑肌细胞(VSMCs)中α1-AR介导的钙动员,较单独去甲肾上腺素组细胞内钙水平降低63%[2] - 浓度高达1 μM时,对α2-AR或β-AR亚型无显著结合活性,证实其α1-AR选择性[2] |
| 体内研究 (In Vivo) |
特拉唑嗪对运动活动和僵直症产生剂量依赖性的完全抑制作用。脑室内注射该拮抗剂可保护纹状体和大脑皮质 α1 受体,但不能保护纹状体或皮质 D1 受体免受 N-乙氧基羰基-2-乙氧基-1,2-二羟基喹啉体内烷基化。心室内注射特拉唑嗪还会导致体温过低和呼吸频率降低,表明交感神经流出减少。特拉唑嗪不会损害水平钢丝测试的表现或游泳测试中协调运动的能力。 Terazosin 在裸鼠中显着抑制血管内皮生长因子诱导的血管生成,IC50 为 7.9 μM,表明其具有比细胞毒作用更有效的抗血管生成作用。
在自发性高血压大鼠(SHR)中,口服盐酸特拉唑嗪(1–10 mg/kg,每日一次)剂量依赖性降低收缩压。5 mg/kg剂量时,收缩压降低28 mmHg,舒张压降低21 mmHg,药效持续24小时[1] - 在睾酮诱导的良性前列腺增生(BPH)大鼠中,盐酸特拉唑嗪(5 mg/kg,口服,每日一次,连续4周)使前列腺重量减轻41%,改善排尿功能(排尿量增加38%,排尿频率减少29%)[3] - 在雄性Wistar大鼠中,静脉注射盐酸特拉唑嗪(0.5 mg/kg)诱导剂量依赖性血管舒张,15分钟内肠系膜血流量增加47%[2] |
| 酶活实验 |
α1-AR放射性配体结合实验:从大鼠大脑皮层(总α1-AR)或过表达人α1A/α1B/α1D亚型的HEK293细胞制备膜组分,膜组分与[3H]-哌唑嗪(特异性α1-AR配体)及系列浓度的盐酸特拉唑嗪(0.1–100 nM)在25°C孵育60分钟。真空过滤去除未结合配体,液体闪烁计数测量结合放射性,采用竞争性结合方程计算Ki值[2]
- 钙动员实验:用钙敏感荧光指示剂负载VSMCs,经盐酸特拉唑嗪(0.05–50 nM)预处理20分钟后,用去甲肾上腺素(1 μM)刺激,实时记录荧光强度,从钙信号抑制的剂量-反应曲线推导IC50值[2] |
| 细胞实验 |
本研究中使用了PC-3细胞和人良性前列腺细胞的原代培养物。使用3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四氮唑测定法和乳酸脱氢酶释放反应检测细胞毒性作用。在裸鼠模型中测定体内血管生成作用,然后进行组织学检查和血红蛋白检测定量。在培养的人脐静脉内皮细胞中进行了细胞迁移、增殖和管形成的体外测定。结果terazosin/特拉唑嗪可诱导PC-3和人类良性前列腺细胞产生细胞毒性,IC50超过100微M。末端脱氧核苷酸转移酶脱氧尿苷三磷酸缺口末端标记和乳酸脱氢酶释放反应阳性与特拉唑嗪诱导的细胞毒性有关,表明细胞凋亡和坏死死亡。此外,特拉唑嗪作用引起的细胞毒性不是喹唑啉基结构的常见特征。特拉唑嗪显著抑制血管内皮生长因子诱导的裸鼠血管生成,IC50为7.9微M。,表明其抗血管生成作用比细胞毒性作用更强。特拉唑嗪还有效地抑制了血管内皮生长因子诱导的培养的人脐静脉内皮细胞的增殖和管形成(IC50分别为9.9和6.8微摩尔)。
结论:我们的数据表明,terazosin/特拉唑嗪通过抑制内皮细胞的增殖和管形成显示出直接的抗血管生成活性。这一作用可能部分解释了特拉唑嗪的体内抗肿瘤潜力[3]。
在当前的研究中,采用了各种鉴定技术来确定细胞毒性作用的作用方式。通过末端脱氧核苷酸转移酶脱氧尿苷三磷酸缺口末端标记,可以原位鉴定凋亡细胞。 PC-3细胞用100μM特拉唑嗪处理12小时后,结果显示阳性反应。 血管平滑肌收缩实验:将大鼠主动脉条(2–3 mm长)置于含Krebs-Ringer缓冲液的器官浴中(37°C,通氧),用去甲肾上腺素(1 μM)预收缩后,加入累积浓度的盐酸特拉唑嗪(0.1–100 nM)。通过等长换能器记录收缩幅度,计算相对于预收缩水平的抑制率[1] - 前列腺基质细胞增殖实验:将WPMY-1细胞以4×103个细胞/孔接种到96孔板,培养24小时。用盐酸特拉唑嗪(1–20 μM)和睾酮(10 nM)处理72小时,MTT法检测570 nm处吸光度,确定增殖抑制率[3] - 细胞周期分析:用盐酸特拉唑嗪(10 μM)处理WPMY-1细胞48小时,70%乙醇固定,碘化丙啶染色后流式细胞术分析,量化细胞周期分布[3] |
| 动物实验 |
Dissolved in water; 0.05 mg/kg; oral gavage
Mice terazosin, a water-soluble alpha 1 antagonist that can be administered in high doses intraventricularly was used to study the relationship between brain alpha 1 adrenoceptor neurotransmission and behavioral activation in the mouse. The antagonist was found to produce a dose-dependent, complete inhibition of motor activity and catalepsy which were reversed preferentially by coinfusion of an alpha 1 agonist (phenylephrine) compared to a D1 (SKF38393) or a D2 agonist, (quinpirole). Blockade of central beta-1 (betaxolol), alpha-2 (RX821002) or beta-2 (ICI 118551) adrenoceptors had smaller or non-significant effects. Terazosin's selectivity for alpha 1 receptors versus dopaminergic receptors was verified under the present conditions by showing that the intraventricularly administered antagonist protected striatal and cerebral cortical alpha 1 receptors but not striatal or cortical D1 receptors from in vivo alkylation by N-ethoxycarbonyl-2-ethoxy-1, 2-dihydroxyquinoline. That its effect was due to blockade of brain rather than peripheral receptors was shown by the finding that intraperitoneal doses of terazosin three to 66 times greater than the maximal intraventricular dose produced less behavioral inhibition. Intraventricular terazosin also produced hypothermia and a reduced respiratory rate suggestive of a reduced sympathetic outflow. However, external heat did not affect the inactivity, and captopril, a hypotensive agent, did not mimic it. Terazosin did not impair performance on a horizontal wire test or the ability to make co-ordinated movements in a swim test suggesting that its activity-reducing effect was not due to sedation and may have a motivational or sensory gating component. It is concluded that central alpha 1-noradrenergic neurotransmission is required for behavioral activation to environmental change in the mouse and may operate on sensorimotor and motivational processes.[2] Hypertensive rat model: Male SHR (12–14 weeks old, n=7/group) were randomly divided into vehicle (0.5% carboxymethylcellulose sodium) and Terazosin HCl groups (1, 5, 10 mg/kg). The drug was administered orally once daily for 14 days. Systolic and diastolic blood pressure were measured by tail-cuff plethysmography every 3 days. At the end of treatment, aortic tissues were collected for α1-AR binding assay [1] - BPH rat model: Male Sprague-Dawley rats (8 weeks old) were subcutaneously injected with testosterone propionate (5 mg/kg/week) for 4 weeks to induce BPH. Rats were then divided into vehicle and Terazosin HCl (5 mg/kg, p.o.) groups (n=8/group), treated once daily for another 4 weeks. Urinary parameters (voiding volume, frequency) were recorded weekly. After euthanasia, prostates were excised, weighed, and processed for histological examination [3] - Vasodilation model: Male Wistar rats (250–300 g) were anesthetized, and a Doppler flow probe was placed around the mesenteric artery. Terazosin HCl (0.1–1 mg/kg) was administered intravenously, and mesenteric blood flow was recorded continuously for 60 min [2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Approximately 90%. Approximately 10% of the oral dose is excreted unchanged in the urine and approximately 20% is excreted in the feces. 40% of the total dose is eliminated in urine and 60% of the total dose is eliminated in the feces. 25L to 30L. Plasma clearance is 80mL/min and renal clearance is 10mL/min. Metabolism / Metabolites The majority of terazosin is hepatically metabolized. The metabolites recovered include 6-O-demethyl terazosin, 7-O-methyl terazosin, a piperozine derivative, and a diamine derivative. Hepatic. One of the four metabolites identified (piperazine derivative of terazosin) has antihypertensive activity. Route of Elimination: Approximately 10% of an orally administered dose is excreted as parent drug in the urine and approximately 20% is excreted in the feces. Half Life: 12 hours Biological Half-Life Terazosin has a mean half life 12 hours though this can be as high as 14 hours in patients over 70 years and as low as 11.4 hours in patients 20 to 39 years old. Oral absorption: In beagle dogs, oral administration of Terazosin HCl (10 mg/kg) results in a maximum plasma concentration (Cmax) of 380 ng/mL, time to reach Cmax (Tmax) of 1.8 h, and oral bioavailability (F) of 65% [3] - Half-life: The elimination half-life (t1/2) is 12.3 h in humans (oral), 10.7 h in dogs, and 8.9 h in rats [3] - Plasma protein binding: Terazosin HCl exhibits 90% plasma protein binding in human plasma and 88% in rat plasma, as determined by equilibrium dialysis [2] - Tissue distribution: After oral administration to rats, Terazosin HCl distributes widely in tissues, with high concentrations in the prostate, vasculature, and kidney (prostate/plasma ratio = 3.2 at 2 h post-administration) [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Terazosin selectively and competitively inhibits vascular postsynaptic alpha(1)-adrenergic receptors, resulting in peripheral vasodilation and a reduction of vascular resistance and blood pressure. Unlike the nonselective alph-adrenergic blockers phenoxybenzamine and phentolamine, terazosin does not block presynaptic alpha(2)-receptors and, hence, does not cause reflex activation of norepinephrine release to produce reflex tachycardia. Hepatotoxicity Terazosin has been associated with a low rate of serum aminotransferase elevations that in controlled trials was no higher than with placebo therapy. These elevations were transient and did not require dose modification. Instances of serum enzyme elevations, but no instances of clinically apparent acute liver injury with jaundice due to terazosin, have been published. Furthermore, product labels do not include discussion of hepatic toxicity. Cholestatic hepatitis and jaundice have been reported with other alpha-adrenergic blockers. Thus, acute symptomatic liver injury due to terazosin must be exceedingly rare if it occurs at all. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because no information is available on the use of terazosin during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information in nursing mothers was not found as of the revision date. However, the pharmacologically similar drug prazosin does not affect serum prolactin concentration in patients with hypertension. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding 90-94%. Acute toxicity: Single oral administration of Terazosin HCl up to 200 mg/kg in mice and rats does not cause mortality or obvious clinical toxicity (e.g., hypotension, lethargy) within 14 days [2] - Repeated-dose toxicity: Rats treated with Terazosin HCl (5–20 mg/kg, p.o., once daily for 28 days) show no significant changes in serum ALT, AST, BUN, or creatinine levels. Histological examination of liver, kidney, prostate, and heart tissues reveals no pathological abnormalities [3] - Orthostatic hypotension: In conscious SHR, Terazosin HCl (10 mg/kg, p.o.) causes a transient 15 mmHg decrease in standing systolic blood pressure, which resolves within 4 hours [1] |
| 参考文献 | |
| 其他信息 |
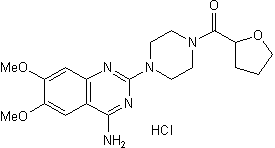
Terazosin Hydrochloride is the hydrochloride salt form of terazosin, a quinazoline derivative with adrenergic antagonistic property. Terazosin hydrochloride selectively inhibits alpha-1 adrenergic receptors, resulting in vasodilation leading to decreased peripheral vascular resistance and a reduced venous return to the heart as well as decreased urethral resistance, which potentially improving urine flow and symptoms related to benign prostatic hyperplasia. In addition, terazosin decreases low-density lipoproteins (LDL) and triglycerides while increasing the concentration of high-density lipoproteins (HDL).
See also: Terazosin (has active moiety). We had previously reported that systemic overexpression of the α1B-adrenergic receptor (AR) in a transgenic mouse induced a neurodegenerative disease that resembled the parkinsonian-like syndrome called multiple system atrophy (MSA). We now report that our mouse model has cytoplasmic inclusion bodies that colocalize with oligodendrocytes and neurons, are positive for α-synuclein and ubiquitin, and therefore may be classified as a synucleinopathy. α-Synuclein monomers as well as multimers were present in brain extracts from both normal and transgenic mice. However, similar to human MSA and other synucleinopathies, transgenic mice showed an increase in abnormal aggregated forms of α-synuclein, which also increased its nitrated content with age. However, the same extracts displayed decreased phosphorylation of α-synuclein. Other traits particular to MSA such as Purkinje cell loss in the cerebellum and degeneration of the intermediolateral cell columns of the spinal cord also exist in our mouse model but differences still exist between them. Interestingly, long-term therapy with the α1-AR antagonist, terazosin, resulted in protection against the symptomatic as well as the neurodegeneration and α-synuclein inclusion body formation, suggesting that signaling of the α1B-AR is the cause of the pathology. We conclude that overexpression of the α1B-AR can cause a synucleinopathy similar to other parkinsonian syndromes.[1] Terazosin HCl is a quinazoline-derived selective α1-adrenergic receptor antagonist, clinically approved for the treatment of hypertension and benign prostatic hyperplasia (BPH) [3] - Its mechanism of action involves competitive binding to α1-ARs in vascular smooth muscle and prostate stroma, inhibiting norepinephrine-induced contraction. This leads to vasodilation (reducing blood pressure) and relaxation of prostate smooth muscle (improving urinary flow) [1] - Terazosin HCl shows similar affinity for all α1-AR subtypes (α1A, α1B, α1D), contributing to its dual efficacy in hypertension and BPH [2] - The drug has a long elimination half-life, allowing once-daily administration, which improves patient compliance [3] - It does not significantly affect heart rate or cardiac output at therapeutic doses, minimizing cardiovascular side effects [1] |
| 分子式 |
C19H26CLN5O4
|
|
|---|---|---|
| 分子量 |
423.9
|
|
| 精确质量 |
423.17
|
|
| 元素分析 |
C, 53.84; H, 6.18; Cl, 8.36; N, 16.52; O, 15.10
|
|
| CAS号 |
63074-08-8
|
|
| 相关CAS号 |
Terazosin hydrochloride dihydrate; 70024-40-7; (R)-Terazosin; 109351-34-0; (S)-Terazosin; 109351-33-9; Terazosin; 63590-64-7
|
|
| PubChem CID |
44383
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
664.5±65.0 °C at 760 mmHg
|
|
| 闪点 |
355.7±34.3 °C
|
|
| 蒸汽压 |
0.0±2.0 mmHg at 25°C
|
|
| 折射率 |
1.636
|
|
| LogP |
-0.96
|
|
| tPSA |
103.04
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
8
|
|
| 可旋转键数目(RBC) |
4
|
|
| 重原子数目 |
29
|
|
| 分子复杂度/Complexity |
544
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O=C(N1CCN(C2=NC(N)=C3C=C(OC)C(OC)=CC3=N2)CC1)C4OCCC4.[H]Cl
|
|
| InChi Key |
IWSWDOUXSCRCKW-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C19H25N5O4.ClH/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14;/h10-11,14H,3-9H2,1-2H3,(H2,20,21,22);1H
|
|
| 化学名 |
[4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]-(oxolan-2-yl)methanone;hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3590 mL | 11.7952 mL | 23.5905 mL | |
| 5 mM | 0.4718 mL | 2.3590 mL | 4.7181 mL | |
| 10 mM | 0.2359 mL | 1.1795 mL | 2.3590 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04551040 | Active Recruiting |
Drug: Terazosin | Healthy | University of Iowa | March 26, 2021 | Phase 1 |
| NCT04760860 | Not yet recruiting | Drug: Terazosin Hydrochloride Other: Placebo |
Dementia With Lewy Bodies | Qiang Zhang | October 2024 | Phase 1 Phase 2 |
| NCT04386317 | Recruiting | Drug: Terazosin | REM Sleep Behavior Disorder Pre-motor Parkinson's Disease |
Cedars-Sinai Medical Center | November 1, 2020 | Phase 2 |
| NCT05109364 | Recruiting | Drug: Terazosin therapy | REM Sleep Behavior Disorder Pre-motor Parkinson's Disease |
Cedars-Sinai Medical Center | September 23, 2022 | Phase 2 |
| NCT05855577 | Not yet recruiting | Drug: Terazosin | Parkinson Disease Gait Analysis Metabolic Disease |
I.R.C.C.S. Fondazione Santa Lucia |
December 2023 | Phase 4 |
 |
|---|
 |
 |