| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
CSF-1R (IC50 = 1 nM); c-Kit (IC50 = 3.2 μM); PDGFRβ (IC50 = 4.8 μM); Flt3 (IC50 = 9.1 μM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:在骨髓源性巨噬细胞 (BMDM) 中,BLZ945 特异性抑制 CSF-1 依赖性增殖,EC50 为 67nM,并降低 CSF-1R 磷酸化。 BLZ945 阻断巨噬细胞和神经胶质瘤细胞之间对彼此存活、增殖和/或极化的相互作用,从而促进肿瘤发生。激酶测定:BLZ945 是一种有效的、口服生物活性的、选择性的 CSF-1R(集落刺激因子 1 受体)抑制剂,IC50 为 1 nM,对其最接近的受体酪氨酸激酶同源物的选择性超过 1000 倍。细胞测定:使用MTT细胞增殖试剂盒测定细胞生长速率。简而言之,将细胞一式三份接种在 96 孔板中:神经胶质瘤细胞系每孔 1,000 个细胞,BMDM 和 CRL-2467 每孔 5 x 1,000 个细胞,HUVEC 和 HBMEC 细胞系每孔 2.5 x 1,000 个细胞。对于所有实验,介质每 48 小时更换一次。细胞在存在或不存在 6.7–6,700 nM BLZ945 或 8 μg/mL CSF-1R 中和抗体的情况下生长。 BMDM 和 CRL-2467 细胞分别补充 10 ng/mL 和 30 ng/mL 重组小鼠 CSF-1。根据制造商的方案,使用读板器通过比色分析检测 MTT 底物的还原,并在 SpectraMax 340pc 读板器上在 595 nm 和 750 nm 处进行测量。
|
| 体内研究 (In Vivo) |
在患有神经胶质瘤的小鼠中,BLZ945 通过抑制 CSF-1R 来阻止肿瘤进展并显着提高生存率。 BLZ945 还在体内抑制患者源性原神经肿瘤球和细胞系的原位肿瘤生长。 BLZ945(200 mg/kg,口服)可降低小鼠乳腺肿瘤病毒驱动的多瘤病毒中 T 抗原 (MMTV-PyMT) 乳腺癌模型和表达角蛋白 14 的人乳头瘤病毒 16 型 (K14-HPV- 16)宫颈癌转基因模型。
|
| 酶活实验 |
BLZ945 是一种有效的、口服生物活性的、选择性的 CSF-1R(集落刺激因子 1 受体)抑制剂,IC50 为 1 nM,对其最接近的受体酪氨酸激酶同源物的选择性超过 1000 倍。
BLZ945, CSF-1R酪氨酸激酶的高选择性小分子抑制剂(比其他酪氨酸激酶高3200倍);参考文献27)。在体外阻断实验中,将BLZ945和GW2580分别溶于10 mmol/L和1 mmol/L的DMSO中制备原液。在体内治疗方面,根据先前的研究,BLZ945溶解在20% Captisol中,剂量为16 mg/mL,每日灌胃剂量为200 mg/kg。[3] 细胞因子分析:在卡罗林斯卡大学医院的核心设施中,通过27参数Luminex多路试验 分析从SK-N-BE(2)、SK-N-AS或SK-N-FI神经母细胞瘤细胞系中收集的培养基或上清液中的细胞因子含量。采用ELISA法测定中药中人或鼠M-CSF (CSF-1)的浓度。[3] |
| 细胞实验 |
CD34+造血祖细胞的分化[3]
采用含有50 ng/mL GM-CSF和5 ng/mL tnf - α的900 μL培养基,24孔板培养CD34+细胞。或者,从上述三种人类神经母细胞瘤细胞系中收集的上清液以2:1的比例添加到祖细胞中。以培养液中维持的CD34+细胞为对照。为了阻断CSF-1R信号,将Sotuletinib (BLZ945) (500 nmol/L)添加到细胞因子成熟的细胞中或细胞因子与SK-N-BE(2)结合的上清液中。7天后,通过清洗和轻轻刮拭收集所有细胞,并通过流式细胞术或基于cfse的t细胞增殖试验评估细胞的表型和功能。 人单核细胞-肿瘤共培养[3] 原代人单核细胞与人神经母细胞瘤细胞系共培养。简而言之,将单核细胞与4×105 SK-N-BE(2)、SK-N-AS或6×105 SK-N-FI神经母细胞瘤细胞在3ml培养基中的6孔板中共培养。未培养肿瘤细胞的单核细胞作为对照。64小时后,通过大力清洗细胞,然后轻轻刮板收获细胞。流式细胞术评价细胞表型变化,用微珠和质谱柱对HLA-DR+细胞进行分选。为了研究CSF-1R的作用,将500 μmol/L Sotuletinib (BLZ945) 或1 μmol/L GW2580加入到单核细胞或共培养中。 小鼠骨髓细胞的分化[3] 抑制性骨髓细胞是根据先前描述的方案,从负基因型TH-MYCN小鼠的骨髓细胞中诱导的。简言之,将1×106分离的骨髓细胞在NHO2A肿瘤条件培养基(中药,1:1稀释至新鲜培养基)存在的6孔板中培养。作为对照,细胞在新鲜培养基或M-CSF (20 ng/mL)中培养。为了阻断CSF-1R信号,在培养物中加入1 μmol/L的Sotuletinib (BLZ945) 或GW2580,并加入DMSO作为对照。4天后,通过收集浮细胞和仔细刮拭孔贴壁细胞的方法收获细胞,随后进行流式细胞术分析或t细胞抑制实验。 |
| 动物实验 |
Mice: Volumes of tumors are measured with calipers using the following formula: volume=(width)2×length/2. 56–63 day old female mice are dosed with 200 mg/kg of sotuletinib or 20% Captisol vehicle in MMTV-PyMT mouse studies. The mice are randomized into groups according to the sizes of their tumors. Tumor volumes are measured twice a week, and the dosage is given orally via gavage once a day. Rat IgG control or 5A1 rat anti-mouse CSF1 neutralizing antibody is injected intraperitoneally every five days at a dose of 10 mg/kg. Formalin-fixed paraffin-embedded lungs in MMTV-PyMT transgenic mice are serially sectioned and stained with hematoxylin and eosin to determine pulmonary metastasis. Tumor regions are rated based on size (tumor diameter), tumor burden (total tumor area divided by total lung area), and the total number of individual metastases counted in a single-blind manner. To get the final value, these values are averaged over the whole lung depth.
Orthotopic allograft models[2] 6–7 wk old female FVB/NJ mice and 6–7 wk old female BALB/c nude mice (CAnN.Cg-Foxn1nu/Crl) were used. For the mammary tumor virus-driven Polyoma middle T antigen (MMTV-PyMT) orthotopic allograft model, spontaneous tumors from 10–13 wk old female transgenic MMTV-PyMT mice were pooled and enzymatically digested with Liberase TM (Roche). The resultant single-cell suspension was then immediately injected orthotopically at the indicated cell dosage into a single mammary fat pad of syngeneic female FVB/NJ recipient mice. For the CD45 allotype study, spontaneous tumors from 10–13 wk old female MMTV-PyMT transgenic mice were harvested by blunt dissection and divided into 3 mm cubes. A small incision was made in the mammary fat pad of female BALB/c nude recipient mice and 2 tumor samples were placed inside the fat pad and sealed with surgical staples. After 5 d, the wound was reopened and the tumor samples retrieved. Tumors were digested and analyzed as described below. Donor and recipient mice were treated with either Sotuletinib (BLZ945) or vehicle for 5 d prior to resection and implantation as described below. CSF1-signaling antagonist pharmacological study in spontaneous tumor models[2] Tumors were measured using calipers and volumes calculated based on the formula: volume = (width)2 × length/2. In MMTV-PyMT mouse studies, 56–63 d old female mice were randomized into groups based on tumor volumes and dosed with either 20% Captisol® vehicle or 200 mg/kg Sotuletinib (BLZ945) . Dosing was administered by oral gavage once daily and tumor volumes were measured twice weekly. 5A1 rat anti-mouse CSF1 neutralizing antibody or rat IgG control was dosed at 10 mg/kg by intraperitoneal injection every 5 d. To calculate pulmonary metastasis in MMTV-PyMT transgenic mice, formalin-fixed paraffin-embedded lungs were serially sectioned and stained with hematoxylin and eosin (H&E). Tumor regions were scored by tumor burden (total tumor area divided by total lung area), size (tumor diameter), and according to the total number of individual metastases counted in a single-blind fashion. These values were averaged across the entire depth of the lung to obtain the final value. For K14-HPV16 mouse studies, female mice were given slow release 17β-estradiol pellets every 2 mo to induce squamous carcinogenesis in the cervical and vaginal epithelium.43,44 Mice were randomized at 6 mo of age at the reported onset of cervical cancer and treated with Sotuletinib (BLZ945) for a 1 mo duration. To determine cervical tumor volume in K14-HPV16 transgenic mice, formalin-fixed paraffin-embedded cervix tissues and neoplasms were serially sectioned, scored for tumor area in a single-blind fashion, and the values multiplied by the tumor depth. |
| 参考文献 |
|
| 其他信息 |
Sotuletinib is an orally bioavailable inhibitor of colony stimulating factor 1 receptor (CSF-1R; CSF1R), with potential antineoplastic activity. CSF1R inhibitor BLZ945 selectively binds to CSF1R expressed on tumor-associated macrophages (TAMs), blocks the activity of CSF1R, and inhibits CSF1R-mediated signal transduction pathways. This inhibits the activity and proliferation of TAMs, and reprograms the immunosuppressive nature of existing TAMs. Altogether, this reduces TAM-mediated immune suppression in the tumor microenvironment, re-activates the immune system, and improves anti-tumor cell responses mediated by T-cells. CSF1R, also known as macrophage colony-stimulating factor receptor (M-CSFR) and CD115 (cluster of differentiation 115), is a cell-surface receptor for its ligand, colony stimulating factor 1 (CSF1); this receptor is overexpressed by TAMs in the tumor microenvironment, and plays a major role in both immune suppression and the induction of tumor cell proliferation.
Purpose: Neuroblastoma is the most common extracranial solid cancer type in childhood, and high-risk patients have poor prognosis despite aggressive multimodal treatment. Neuroblastoma-driven inflammation contributes to the induction of suppressive myeloid cells that hamper efficient antitumor immune responses. Therefore, we sought to enhance antitumor immunity by removing immunosuppression mediated by myeloid cells. Experimental Design: The prognostic values of myeloid cells are demonstrated by analyzing genomic datasets of neuroblastoma patients. The impact of tumor-derived factors on myelopoiesis and local induction of suppressive myeloid cells is dissected by in vitro culture models using freshly isolated human CD34+ hematopoietic stem cells, primary human monocytes, and murine bone marrow cells. To test the therapeutic efficacy of BLZ945 as a monotherapy or in combination with checkpoint inhibitors, we used a transgenic murine model (TH-MYCN) that develops aggressive spontaneous neuroblastoma. Results: We report that infiltrating CSF-1R+ myeloid cells predict poor clinical outcome in patients with neuroblastoma. In vitro, neuroblastoma-derived factors interfere with early development of myeloid cells and enable suppressive functions on human monocytes through M-CSF/CSF-1R interaction. In a transgenic mouse model (TH-MYCN) resembling high-risk human neuroblastoma, antagonizing CSF-1R with a selective inhibitor (BLZ945) modulates the induction of human and murine suppressive myeloid cells and efficiently limit tumor progression. While checkpoint inhibitors are insufficient in controlling tumor growth, combining BLZ945 with PD-1/PD-L1 blocking antibodies results in superior tumor control. Conclusions: Our results demonstrate the essential role of CSF-1R signaling during the induction of suppressive myeloid cells and emphasize its clinical potential as an immunotherapy for human cancers. [3] Increased numbers of tumor-infiltrating macrophages correlate with poor disease outcome in patients affected by several types of cancer, including breast and prostate carcinomas. The colony stimulating factor 1 receptor (CSF1R) signaling pathway drives the recruitment of tumor-associated macrophages (TAMs) to the neoplastic microenvironment and promotes the differentiation of TAMs toward a pro-tumorigenic phenotype. Twelve clinical trials are currently evaluating agents that target the CSF1/CSF1R signaling pathway as a treatment against multiple malignancies, including breast carcinoma, leukemia, and glioblastoma. The blockade of CSF1R signaling has been shown to greatly decrease the number of macrophages in a tissue-specific manner. However, additional mechanistic insights are needed in order to understand how macrophages are depleted and the global effects of CSF1R inhibition on other tumor-infiltrating immune cells. Using BLZ945, a highly selective small molecule inhibitor of CSF1R, we show that CSF1R inhibition attenuates the turnover rate of TAMs while increasing the number of CD8+ T cells that infiltrate cervical and breast carcinomas. Specifically, we find that BLZ945 decreased the growth of malignant cells in the mouse mammary tumor virus-driven polyomavirus middle T antigen (MMTV-PyMT) model of mammary carcinogenesis. Furthermore, we show that BLZ945 prevents tumor progression in the keratin 14-expressing human papillomavirus type 16 (K14-HPV-16) transgenic model of cervical carcinogenesis. Our results demonstrate that TAMs undergo a constant turnover in a CSF1R-dependent manner, and suggest that continuous inhibition of the CSF1R pathway may be essential to maintain efficacious macrophage depletion as an anticancer therapy.[2] Glioblastoma multiforme (GBM) comprises several molecular subtypes, including proneural GBM. Most therapeutic approaches targeting glioma cells have failed. An alternative strategy is to target cells in the glioma microenvironment, such as tumor-associated macrophages and microglia (TAMs). Macrophages depend on colony stimulating factor-1 (CSF-1) for differentiation and survival. We used an inhibitor of the CSF-1 receptor (CSF-1R) to target TAMs in a mouse proneural GBM model, which significantly increased survival and regressed established tumors. CSF-1R blockade additionally slowed intracranial growth of patient-derived glioma xenografts. Surprisingly, TAMs were not depleted in treated mice. Instead, glioma-secreted factors, including granulocyte-macrophage CSF (GM-CSF) and interferon-γ (IFN-γ), facilitated TAM survival in the context of CSF-1R inhibition. Expression of alternatively activated M2 markers decreased in surviving TAMs, which is consistent with impaired tumor-promoting functions. These gene signatures were associated with enhanced survival in patients with proneural GBM. Our results identify TAMs as a promising therapeutic target for proneural gliomas and establish the translational potential of CSF-1R inhibition for GBM.[1] |
| 精确质量 |
434.11793
|
|---|---|
| 元素分析 |
C, 50.96; H, 5.13; Cl, 15.04; N, 11.89; O, 10.18; S, 6.80
|
| CAS号 |
2222138-40-9
|
| 相关CAS号 |
Sotuletinib;953769-46-5;Sotuletinib hydrochloride;2222138-31-8
|
| PubChem CID |
141759984
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| LogP |
125Ų
|
| InChi Key |
ZIHWHYXECXSBNA-LVVRIOTCSA-N
|
| InChi Code |
InChI=1S/C20H22N4O3S.2ClH/c1-21-19(26)16-10-13(8-9-22-16)27-12-6-7-15-18(11-12)28-20(24-15)23-14-4-2-3-5-17(14)25;;/h6-11,14,17,25H,2-5H2,1H3,(H,21,26)(H,23,24);2*1H/t14-,17-;;/m1../s1
|
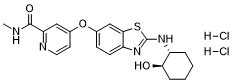
| 化学名 |
4-((2-(((1R,2R)-2-hydroxycyclohexyl)amino)benzo[d]thiazol-6-yl)oxy)-N-methylpicolinamide dihydrochloride
|
| 别名 |
Sotuletinib dihydrochloride; BLZ945; Sotuletinib hydrochloride; 2222138-31-8; Sotuletinib (hydrochloride); 4-((2-(((1R,2R)-2-Hydroxycyclohexyl)amino)benzo[d]thiazol-6-yl)oxy)-N-methylpicolinamide hydrochloride; Sotuletinib HCl?; BLZ945 HCl; BLZ945 HYDROCHLORIDE; BLZ 945; BLZ-945.Sotuletinib;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。