| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
EGFR
|
|---|---|
| 体外研究 (In Vitro) |
吉非替尼二盐酸盐(0.01–0.1 μM,72 小时)可增强贴壁依赖性生长和增殖,增强 ERK 信号传导,并增加受体的磷酸酪氨酸负载 [2]。使用吉非替尼二盐酸盐(1-2 μM,72 小时)观察到 EGFRvIII 磷酸酪氨酸负载、EGFRvIII 介导的增殖和锚定非依赖性生长显着降低 [2]。通过 STAT6 依赖性信号通路,吉非替尼二氯化物(0.62 μM,24-72 小时)抑制 IL-13 生成的 RAW 264.7 细胞的 M2 样极化 [3]。吉非替尼二氯化物(0.62 μM,72 小时)可抑制 M2 样巨噬细胞促进的侵袭和迁移 [3]。在 NSCLC 细胞系(H3255 和 HCC827 细胞)中,吉非替尼二氯化物(0–10 μM,72 小时)会导致细胞凋亡(诱导 BIM 蛋白)[4]。在 HCC827 和 A549 细胞中,gefitinib diHClide(100 nM,24 h)促进细胞外囊泡 (EV) 的吸收并抑制巨胞饮作用 [6]。顺铂耐药的 wtEGFR NSCLC 细胞系 H358R 和 A549R 的生长更容易受到吉非替尼二盐酸化物(1.5–60 μM,48 小时)的抑制[7]。
|
| 体内研究 (In Vivo) |
吉非替尼二盐酸盐(口服,75 mg/kg/d,21 天)可抑制 LLC 小鼠转移模型中巨噬细胞的 M2 样极化 [3]。吉非替尼二盐酸盐(口服,第一周 75 mg/kg,每天一次,每周 5 天)消除小叶增生和癌症中 HER2 和 HER3 的磷酸化以及通过 MAPK 和 Akt 的信号传导,增加脾细胞、淋巴中的 MAPK 活性和细胞因子产生节点[5]。吉非替尼二盐酸盐(150 mg/kg,口服,每日)可提高顺铂在 H358R 异种移植物中的抗癌活性 [7]。
|
| 细胞实验 |
蛋白质印迹分析[2]
细胞类型: NR6wtEGFR、NR6W 和 NR6M 测试浓度: 1、10、100 μM 孵育持续时间:5小时 实验结果:抑制EGFR酪氨酸磷酸化。 细胞迁移测定 [3] 细胞类型: LLC 细胞 测试浓度: 0.62 μM 孵育时间:72小时 实验结果:消除M2样巨噬细胞促进LLC的侵袭和迁移。 |
| 动物实验 |
Animal/Disease Models: LLC mouse metastasis model [3]
Doses: 75 mg/kg/d for 21 days. Route of Administration: Oral administration Experimental Results: Reduce the number of lung metastasis nodules, down-regulate the expression of M2 marker genes and the percentage of CD206+ and CD68+ macrophages in tumor tissue. Animal/Disease Models: BALB-NeuT transgenic mouse model [5] Doses: 75 mg/kg in the first week, increasing by 15 mg/kg every other week, one time/day, for 5 days a week, followed by 2 days of no treatment, repeated for 8- 9 weeks. Route of Administration: Oral administration Experimental Results: Treated mice diminished tumor multiplicity from 9.6 to 0.58 (83%) and diminished the number and size of lobules and lobular nodules. |
| 参考文献 |
[1]. Wakeling AE, et al. ZD1839: an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002 Oct 15;62(20):5749-54.
[2]. Pedersen MW, et al. Differential response to gefitinib of cells expressing normal EGFR and the mutant EGFRvIII. Br J Cancer. 2005 Oct 17;93(8):915-23. [3]. Muhammad Tariq, et al. Gefitinib inhibits M2-like polarization of tumor-associated macrophages in Lewis lung cancer by targeting the STAT6 signaling pathway. Acta Pharmacol Sin. 2017 Nov;38(11):1501-1511. [4]. Mark S Cragg, et al. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 2007 Oct;4(10):1681-89; discussion 1690. [5]. Marie P Piechocki, et al. Gefitinib prevents cancer progression in mice expressing the activated rat HER2/neu. Int J Cancer. 2008 Apr 15;122(8):1722-9. [6]. Tomoya Takenaka, et al. Effects of gefitinib treatment on cellular uptake of extracellular vesicles in EGFR-mutant non-small cell lung cancer cells. Int J Pharm. 2019 Dec 15;572:118762. [7]. Amin Li, et al. Gefitinib sensitization of cisplatin-resistant wild-type EGFR non-small cell lung cancer cells. J Cancer Res Clin Oncol. 2020 Jul;146(7):1737-1749. |
| 其他信息 |
The epidermal growth factor receptor (EGFR) is a promising target for anticancer therapy because of its role in tumor growth, metastasis and angiogenesis, and tumor resistance to chemotherapy and radiotherapy. We have developed a low-molecular-weight EGFR tyrosine kinase inhibitor (EGFR-TKI), ZD1839 (Iressa(2) ). ZD1839, a substituted anilinoquinazoline, is a potent EGFR-TKI (IC(50) = 0.033 micro M) that selectively inhibits EGF-stimulated tumor cell growth (IC(50) = 0.054 micro M) and that blocks EGF-stimulated EGFR autophosphorylation in tumor cells. In studies with mice bearing a range of human tumor-derived xenografts, ZD1839 given p.o. once a day inhibited tumor growth in a dose-dependent manner. The level of expression of EGFR did not determine xenograft tumor sensitivity to ZD1839. Long-term ZD1839 (>3 months) treatment of mice bearing A431 xenografts was well tolerated, and ZD1839 completely inhibited tumor growth and induced regression of established tumors. No drug-resistant tumors appeared during ZD1839 treatment, but some tumors regrew after drug withdrawal. These studies indicate the potential utility of ZD1839 in the treatment of many human tumors and indicate that continuous once-a-day p.o. dosing might be a suitable therapeutic regimen. [1]
Epidermal growth factor receptor (EGFR) is frequently amplified and/or mutated in a number of human tumours and abnormal signalling from this receptor is believed to contribute to the malignant phenotype seen in these tumours. Gefitinib is a small molecule inhibitor that specifically binds and inhibits the EGFR tyrosine kinase and has been shown to inhibit the growth, proliferation, survival and invasion of a range of tumour cells overexpressing EGFR. However, clinical response to gefitinib has failed to correlate with EGFR levels and activity, indicating that other molecular mechanisms such as downstream signalling and mutations could be of importance in predicting clinical response. We therefore investigated the effect of the specific EGFR inhibitor gefitinib on the phosphorylation level, signalling and growth of cells expressing the naturally occurring constitutively active EGFR variant EGFRvIII, a low nontransforming level of EGFR and a high transforming level of EGFR. Results show that levels of gefitinib sufficient to suppress EGFR phosphorylations, EGFR-mediated proliferation and EGFR-mediated anchorage-independent growth are not sufficient to inhibit these features in cells expressing EGFRvIII. Furthermore, the data indicate that long-term exposure of EGFRvIII-expressing cells to low concentrations of gefitinib (0.01-0.1 microM) result in increased phosphotyrosine load of the receptor, increased signalling to ERK and stimulation of proliferation and anchorage-independent growth, presumably by inducing EGFRvIII dimerisation. Higher concentrations of gefitinib (1-2 microM), on the other hand, significantly decreased EGFRvIII phosphotyrosine load, EGFRvIII-mediated proliferation and anchorage-independent growth. Further studies are needed to investigate the implications of these important findings in the clinical setting.[2] M2-like polarized tumor-associated macrophages (TAMs) play a pivotal role in promoting cancer cell growth, invasion, metastasis and angiogenesis. The identification of M2-like TAMs during tumor progression is an attractive approach for cancer therapy. In this study, we investigated the relevance of macrophage polarization and the antitumor effect of gefitinib in Lewis Lung cancer (LLC) in vitro and in vivo. Gefitinib at a concentration below 2.5 μmol/L did not cause significant growth inhibition on LLC and RAW 264.7 cell lines and bone marrow-derived macrophage (BMDMs). However, a small concentration of gefitinib (0.62 μmol/L) significantly inhibited IL-13-induced M2-like polarization of macrophages, evidenced by the decreased expression of the M2 surface markers CD206 and CD163, down-regulation of specific M2-marker genes (Mrc1, Ym1, Fizz1, Arg1, IL-10 and CCL2) as well as inhibition of M2-like macrophage-mediated invasion and migration of LLC cells. In RAW 264.7 cells, gefitinib inhibits IL-13-induced phosphorylation of STAT6, which was a crucial signaling pathway in macrophage M2-like polarization. In LLC mice metastasis model, oral administration of gefitinib (75 mg·kg-1·d-1, for 21 d) significantly reduced the number of lung metastasis nodules, down-regulated the expression of M2 marker genes and the percentages CD206+ and CD68+ macrophages in tumor tissues. These results demonstrated that gefitinib effectively inhibits M2-like polarization both in vitro and in vivo, revealing a novel potential mechanism for the chemopreventative effect of gefitinib. [3] |
| 分子式 |
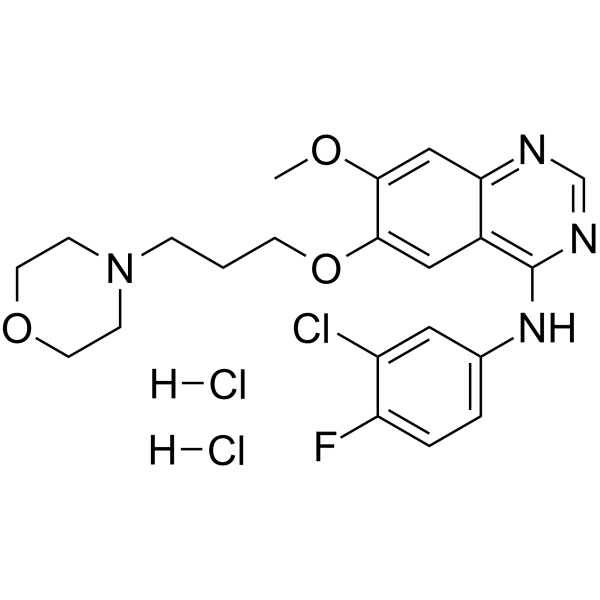
C22H24N4O3FCL.2[HCL]
|
|---|---|
| 分子量 |
519.82424
|
| 精确质量 |
518.105
|
| CAS号 |
184475-56-7
|
| 相关CAS号 |
Gefitinib;184475-35-2
|
| PubChem CID |
19077503
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 沸点 |
628.2ºC at 760 mmHg
|
| 闪点 |
333.7ºC
|
| 蒸汽压 |
4.9E-16mmHg at 25°C
|
| LogP |
5.89
|
| tPSA |
68.74
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
545
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
OHAYARNLYSPHOJ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C22H24ClFN4O3.2ClH/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15;;/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27);2*1H
|
| 化学名 |
N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine;dihydrochloride
|
| 别名 |
184475-56-7; Gefitinib (Dihydrochloride); 4-(3-Chloro-4-fluorophenylamino)-7-methoxy-6-[3-(4-morpholinyl)propoxy]quinazoline dihydrochloride; Gefitinib 2hydrochloride salt; gefitinib dihydrochloride; N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine;dihydrochloride; ZD 1839 Dihydrochloride;ZD-1839 Dihydrochloride;ZD1839 Dihydrochloride; SCHEMBL8208642;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9237 mL | 9.6187 mL | 19.2374 mL | |
| 5 mM | 0.3847 mL | 1.9237 mL | 3.8475 mL | |
| 10 mM | 0.1924 mL | 0.9619 mL | 1.9237 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03292133 | Active Recruiting |
Drug: EGF816 Drug: Gefitinib |
Lung Cancer | Massachusetts General Hospital | October 31, 2017 | Phase 2 |
| NCT03122717 | Active Recruiting |
Drug: Gefitinib Drug: Osimertinib |
Non-Small Cell Lung Cancer | Dana-Farber Cancer Institute | May 9, 2017 | Phase 1 Phase 2 |
| NCT03758287 | Active Recruiting |
Drug: Gefitinib Drug: CT053PTSA |
Non-small Cell Lung Cancer | Sunshine Lake Pharma Co., Ltd. | November 2016 | Phase 1 Phase 2 |
| NCT03849768 | Active Recruiting |
Drug: Gefitinib Drug: HS-10296 |
Non Small Cell Lung Cancer | Jiangsu Hansoh Pharmaceutical Co., Ltd. |
February 1, 2019 | Phase 3 |
| NCT02856893 | Active Recruiting |
Drug: Gefitinib Drug: Osimertinib |
NSCLC | European Organisation for Research and Treatment of Cancer - EORTC |
October 10, 2017 | Phase 2 |