| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
Bcl-W (Ki=1 nM); Bcl-xL (Ki=1 nM); Bcl-2 (Ki=1 nM)
|
|---|---|
| 体外研究 (In Vitro) |
Bcl-2/Bcl-xL 与促凋亡蛋白的相互作用被 ABT-263 破坏,ABT-263 在结构上与 ABT-737 相关。肿瘤的维持、进展和化疗耐药性常常与促存活 Bcl-2 家族成员的过度表达有关。 ABT-263 表现出由 Bcl-2 或 Bcl-xL 过表达提供的防御作用,EC50 值分别为 60 nM 和 20 nM。 ABT-263 抑制最敏感细胞系 (H146) 50% 的生长,EC50 为 110 nM,而最不敏感细胞系 (H82) 则表现出广泛的细胞活性,EC50 为 22 M。两种最耐药的细胞细胞系(H1048 和 H82)也对 ABT-263 具有类似的耐药性,所有四种细胞系(H146、H889、H1963 和 H1417)的 EC50 值均小于 400 nM。
|
| 体内研究 (In Vivo) |
在 H345 异种移植模型中,80% TGI 和 20% 的治疗肿瘤具有显着的抗肿瘤功效,表明肿瘤体积至少减少了 50%。在小细胞肺癌和急性淋巴细胞白血病的异种移植模型中,单独口服 ABT-263 可导致肿瘤总体消退。 ABT-263 显着提高了侵袭性 B 细胞淋巴瘤和多发性骨髓瘤异种移植模型中临床相关治疗方案的疗效,在这些模型中,ABT-263 表现出适度的单药活性或没有单药活性。
|
| 酶活实验 |
ABT-263 对 Bcl-2 家族不同亚型的结合亲和力(Ki 或 IC50)通过竞争性荧光偏振测定来确定。使用以下肽探针/蛋白质对:f-bad (1 nM) 和 Bcl-xL (6 nM)、f-Bax (1 nM) 和 Bcl-2 (10 nM)、f-Bax (1 nM) 和Bcl-w (40 nM)、f-Noxa (2 nM) 和 Mcl-1 (40 nM)、f-Bax (1 nM) 和 Bcl-2-A1 (15 nM)。 Bcl-xL 的结合亲和力也可使用时间分辨荧光共振能量转移测定来确定。 Bcl-xL(1 nM,His 标记)与 200 nM f-Bak、1 nM Tb 标记的抗 His 抗体和 ABT-263 在室温下混合 30 分钟。使用 340/35 nm 激发滤光片和 520/525 (f-Bak) 和 495/510 nm(Tb 标记的抗 His 抗体)发射滤光片在 Envision 酶标仪上测量荧光。
|
| 细胞实验 |
人肿瘤细胞系SCLC细胞系维持在37℃、含有5%CO2的条件下。 SCLC 细胞系在含有 10% 胎牛血清 (FBS)、1% 丙酮酸钠、25 mM HEPES、4.5 g/L 葡萄糖和 1% 青霉素/链霉素的 RPMI 1640 中培养。白血病和淋巴瘤细胞系在补充有 10% FBS 和 1% 青霉素/链霉素的 RPMI 1640 中培养。将细胞 (1-5×10 4) 在 96 孔培养板中用 ABT-263 处理 48 小时,最终体积为 100 μL,并使用 CellTiter Glo 测定评估细胞毒性。测定了 ABT-263 的体外细胞毒性。
|
| 动物实验 |
ABT-263 was dissolved in 60% Phosal 50 PG (w/w), 30% PEG 400 (w/w), 10% ethanol (w/w) and administered orally at its maximum tolerated dose of 100 mg/kg daily × 21 days. ABT-263 was provided to each consortium investigator in coded vials for blinded testing, according to the PPTP's standard operating procedures. CB17SC-M scid−/− female mice were used to propagate subcutaneously implanted kidney/rhabdoid tumors, sarcomas (Ewing, osteosarcoma, rhabdomyosarcoma), neuroblastoma, and non-glioblastoma brain tumors, while BALB/c nu/nu mice were used for glioma models. Human leukemia cells were propagated by intravenous inoculation in female non-obese diabetic (NOD)/scid−/− mice as described previously. [1]
|
| 药代性质 (ADME/PK) |
- In nude mice: After a single oral dose of Navitoclax (ABT-263) (50 mg/kg), the maximum plasma concentration (Cmax) was 8.2 ± 1.5 μg/mL, time to Cmax (Tmax) was 2.0 ± 0.5 hours, and elimination half-life (t1/2) was 6.8 ± 1.2 hours. Oral bioavailability was approximately 45 ± 7% (compared to intravenous administration). The drug distributed widely to tumor tissues, with a tumor/plasma concentration ratio of 3.2 ± 0.4 at 4 hours post-administration[3]
|
| 毒性/毒理 (Toxicokinetics/TK) |
- In pediatric xenograft models: Mice treated with Navitoclax (ABT-263) (25 mg/kg/day) showed mild, reversible thrombocytopenia (platelet count: 85 ± 12 × 10⁹/L vs. 152 ± 18 × 10⁹/L in control, p < 0.05) on day 14, which recovered by day 21. No significant changes in serum ALT, AST, BUN, or creatinine were observed[1]
- In ovarian cancer xenograft models: The combination of Navitoclax (ABT-263) (50 mg/kg) and carboplatin (20 mg/kg) caused no significant increase in toxicity compared to monotherapy. Mice in all groups had similar body weight changes (≤ 10% weight loss), and serum markers of liver (ALT: 45 ± 8 U/L) and kidney (BUN: 18 ± 3 mg/dL) function were within normal ranges[2] - In NSCLC xenograft models: Navitoclax (ABT-263) (30 mg/kg/day) had a plasma protein binding rate of 97 ± 2%. No severe hematological toxicity (neutropenia, anemia) or organ damage was observed in combination with cisplatin. The maximum tolerated dose (MTD) of oral Navitoclax (ABT-263) in nude mice was 60 mg/kg/day (weight loss > 15% at 70 mg/kg)[3] |
| 参考文献 |
|
| 其他信息 |
ABT-263 is a potent (K(i) < 1 nM) small-molecule BH3 mimetic that inhibits the antiapoptotic proteins Bcl-2, Bcl-x(L) and Bcl-w. The structurally related Bcl-2 inhibitor ABT-737 exhibits single-agent preclinical activity against lymphoma, small-cell lung carcinoma, and chronic lymphocytic leukemia and displays synergistic cytotoxicity with chemotherapeutics and radiation.ABT-263 demonstrated in vitro activity against a range of cell lines, with the ALL cell lines showing the greatest sensitivity. ABT-263 demonstrated limited single agent in vivo activity against the PPTP's solid tumor panels but showed significant activity against xenografts in the ALL panel.[1]
To examine the potential of combining Bcl-2 family inhibitors with chemotherapy in ovarian cancer, we evaluated a panel of 27 ovarian cancer cell lines for response to the combination of navitoclax (formerly ABT-263) and paclitaxel or gemcitabine. The majority of cell lines exhibited a greater than additive response to either combination, as determined by the Bliss independence model, and more than 50% of the ovarian cell lines exhibited strong synergy for the navitoclax/paclitaxel combination. To identify biomarkers for tumors likely to respond to this combination, we evaluated the protein levels of intrinsic apoptosis pathway components. Bcl-x(L) seems necessary, but not sufficient, for navitoclax/paclitaxel synergy in vitro, suggesting that exclusion of patients whose tumors have low or undetectable Bcl-x(L) would enrich for patients responsive to the combination. We evaluated Bcl-x(L) levels in ovarian cancer tumor tissue from 40 patients (20 taxane responsive and 20 with poor response to taxane) and found that patients with high Bcl-x(L) were less sensitive to taxane treatment (10 of 12) Bcl-x(L) positive patients, P = 0.014). These data support the use of navitoclax in combination with taxane-based therapy in ovarian cancer patients with high levels of Bcl-x(L).[2] The ability of a cancer cell to avoid apoptosis is crucial to tumorigenesis and can also contribute to chemoresistance. The Bcl-2 family of prosurvival proteins (Bcl-2, Bcl-X(L), Bcl-w, Mcl-1, and A1) plays a key role in these processes. We previously reported the discovery of ABT-263 (navitoclax), a potent small-molecule inhibitor of Bcl-2, Bcl-X(L), and Bcl-w. While navitoclax exhibits single-agent activity in tumors dependent on Bcl-2 or Bcl-X(L) for survival, the expression of Mcl-1 has been shown to confer resistance to navitoclax, most notably in solid tumors. Thus, therapeutic agents that can downregulate or neutralize Mcl-1 are predicted to synergize potently with navitoclax. Here, we report the activity of navitoclax in combination with 19 clinically relevant agents across a panel of 46 human solid tumor cell lines. Navitoclax broadly enhanced the activity of multiple therapeutic agents in vitro and enhanced efficacy of both docetaxel and erlotinib in xenograft models. The ability of navitoclax to synergize with docetaxel or erlotinib corresponded to an altered sensitivity of the mitochondria toward navitoclax, which was associated with the downmodulation of Mcl-1 and/or upregulation of Bim. These data provide a rationale to interrogate these combinations clinically.[3] |
| 分子式 |
C47H57CL3F3N5O6S3
|
|---|---|
| 分子量 |
1047.53459620476
|
| 精确质量 |
1045.25
|
| 元素分析 |
C, 53.89; H, 5.48; Cl, 10.15; F, 5.44; N, 6.69; O, 9.16; S, 9.18
|
| CAS号 |
1093851-28-5
|
| 相关CAS号 |
923564-51-6; 1093851-28-5 (HCl); 2143096-93-7 (Navitoclax-piperazine)
|
| PubChem CID |
46937443
|
| 外观&性状 |
Typically exists as solids
|
| LogP |
13.003
|
| tPSA |
170.42
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
14
|
| 可旋转键数目(RBC) |
16
|
| 重原子数目 |
67
|
| 分子复杂度/Complexity |
1800
|
| 定义原子立体中心数目 |
1
|
| SMILES |
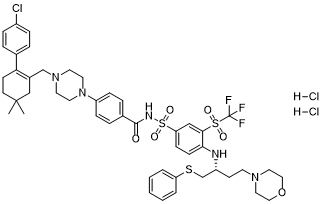
ClC1C=CC(=CC=1)C1CCC(C)(C)CC=1CN1CCN(C2C=CC(C(NS(C3C=CC(=C(C=3)S(C(F)(F)F)(=O)=O)N[C@@H](CSC3C=CC=CC=3)CCN3CCOCC3)(=O)=O)=O)=CC=2)CC1.Cl.Cl
|
| InChi Key |
WDVGRPCSLPVWKC-VROLVAQFSA-N
|
| InChi Code |
InChI=1S/C47H55ClF3N5O6S3.2ClH/c1-46(2)20-18-42(34-8-12-37(48)13-9-34)36(31-46)32-55-22-24-56(25-23-55)39-14-10-35(11-15-39)45(57)53-65(60,61)41-16-17-43(44(30-41)64(58,59)47(49,50)51)52-38(19-21-54-26-28-62-29-27-54)33-63-40-6-4-3-5-7-40;;/h3-17,30,38,52H,18-29,31-33H2,1-2H3,(H,53,57);2*1H/t38-;;/m1../s1
|
| 化学名 |
4-[4-[[2-(4-chlorophenyl)-5,5-dimethylcyclohexen-1-yl]methyl]piperazin-1-yl]-N-[4-[[(2R)-4-morpholin-4-yl-1-phenylsulfanylbutan-2-yl]amino]-3-(trifluoromethylsulfonyl)phenyl]sulfonylbenzamide;dihydrochloride
|
| 别名 |
NAVITOCLAX DIHYDROCHLORIDE; Navitoclax dihydrochloride [USAN]; 1093851-28-5; W8FZ00AY2S; A-855071.3; Navitoclax dihydrochloride (USAN); Navitoclax HCl; 4-(4-((2-(4-Chlorophenyl)-5,5-dimethylcyclohex-1-en-1-yl)methyl)piperazin-1-yl)-N-((4- (((2R)-4-(morpholin-4-yl)-1-(phenylsulfanyl)butan-2-yl)amino)-3- ((trifluoromethyl)sulfonyl)phenyl)sulfonyl)benzamide dihydrochloride;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.9546 mL | 4.7731 mL | 9.5462 mL | |
| 5 mM | 0.1909 mL | 0.9546 mL | 1.9092 mL | |
| 10 mM | 0.0955 mL | 0.4773 mL | 0.9546 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Phase 1/2a Study Evaluating the Safety, Pharmacokinetics, and Efficacy of ABT-263 in Subjects with Small Cell Lung Cancer (SCLC) or other non-hematological malignancies.
CTID: null
Phase: Phase 1, Phase 2 Status: Completed
Date: 2007-05-23