| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
NMDA Receptor
|
|---|---|
| 体外研究 (In Vitro) |
Ro 25-6981是含有NR2B亚基的NMDA受体的选择性和活性依赖性阻断剂。它在结构上与非苯丙地尔相关,对非竞争性拮抗剂如苯环利定或MK-801的已知结合位点没有亲和力Fischer等,1997,Lynch等,2001,Mutel等,1998。在转染了编码NR1C和NR2B亚基的cDNA混合物的爪蟾卵母细胞中,Ro 25-6981可作为有效的拮抗剂。相比之下,在转染NR2A亚基的卵母细胞中,其拮抗NMDA反应的效力几乎低了四个数量级(Fischer et al., 1997)。[1]
|
| 体内研究 (In Vivo) |
当腹膜内给予 Ro 25-6981 (0.39-12.5 mg/kg) 时,6-羟基多巴胺 (6-OHDA) 损伤的大鼠表现出反向旋转;然而,正常大鼠不会表现出任何运动刺激[1]。在大鼠出生后早期发育中,Ro 25-6981(1.3 mg/kg;腹腔注射)显示出年龄和激活依赖性的抗惊厥作用[2]。鞘内注射 Ro 25-6981 (800 µg) 可显着降低术后痛觉过敏,并对大鼠切口疼痛具有相当大的镇痛作用 [3]。
n -甲基- d -天冬氨酸(NMDA)受体拮抗剂在帕金森病(PD)动物模型中具有抗运动和抗运动障碍作用。然而,非选择性抑制NMDA受体在整个中枢神经系统可能导致不希望的影响,如共济失调和精神病。因此,我们研究了Ro 25-6981,一种NMDA受体的活性依赖性拮抗剂,含有NR2B亚基,主要在纹状体中表达。Ro 25-6981诱导6-羟多巴胺(6- ohda)损伤大鼠的对抗性旋转,而不刺激正常大鼠的运动,并逆转1-甲基-4-苯基-1,2,3,6,-四氢吡啶(MPTP)治疗的普通狨猴的帕金森症状。由于绒猴数量较少,Ro 25-6981与载虫之间的差异不显著,但有显著的差异趋势,由Page检验可知。此外,Ro 25-6981增强了左旋多巴在两种动物中的作用,并减弱了长期服用左旋多巴的6- ohda损伤大鼠的最大左旋反应,但不降低总体反应。Ro 25-6981还能增强多巴胺受体激动剂阿波啡、A68930和喹匹罗对6- ohda损伤大鼠的作用。目前的观察结果表明,nr2b选择性NMDA受体拮抗剂在帕金森病的治疗中具有治疗潜力。[1] Ro 25-6981是含有NR2B亚基(NR2B/NMDARs)的NMDA嗜离子型谷氨酸受体的高选择性和活性依赖性拮抗剂。本研究旨在探讨Ro 25-6981给药对发育大鼠生理(单脉冲和成对脉冲皮层半球间诱发电位)和癫痫性脑活动(皮质放电后放电(ADs))的影响。在出生后(P) P12、P18和P25进行硬膜外电极植入动物的电生理实验。该药物以1或3mg/kg的剂量腹腔注射。对照动物注射生理盐水(1ml/kg)。用0.5 ms双相脉冲(强度从0.4 ~ 5mA增加)诱发单半球间反应,用两倍阈值强度诱发双脉冲反应。在8赫兹频率下,通过一系列15秒的1毫秒脉冲来激发ad。首先,采用6次稳定的超过阈值强度的刺激,每隔30分钟重复一次,以确定Ro 25-6981对P12动物ADs的作用时间过程。其次,在所有年龄组的动物中进行了类似的实验,但间隔20分钟,以及进一步的实验,使用从0.2到15 mA的10分钟间隔逐步增加的刺激强度。预处理3 mg/kg(不低于3 mg/kg)的ro25 -9681可显著降低P12和P18动物在高刺激强度下的单次反应幅度。两种剂量都影响P25动物的反应,只有1 mg/kg剂量比3 mg/kg剂量更有效。在任何年龄组中,任何剂量的Ro 25-6981均未影响配对脉冲反应。Ro 25-9681仅对P12动物的ad持续时间有明显影响。1 mg/kg剂量不改变ad持续时间,而3 mg/kg剂量通过反复刺激抑制ad持续时间延长。即使在药物注射后110分钟,这种效果仍然存在。ADs的修改,即逐步增加强度的刺激(间隔10分钟)被用来证明可能对活动的依赖性。在4-mA刺激后立即给予Ro 25-6981(即当大鼠平均经历6次ad时)。3mg /kg剂量可使P12在高刺激强度后ADs缩短。在老年动物中没有显着影响,仅在P25大鼠中观察到ad缩短的趋势。总之,我们的研究结果表明,作为NR2B/NMDARs的选择性拮抗剂,Ro 25-6981在出生后早期表现出年龄依赖性和激活依赖性的抗惊厥作用。相反,ro25 -6981对感觉运动皮层单脉冲刺激引起的生理兴奋性的影响不依赖于年龄。因此,这种化合物可能是一种有用的抗癫痫药,因为它对ad延长的作用可以在单次给药后110分钟观察到。[2] 背景:NR2B亚单位(NMDA受体2B亚单位)在疼痛的产生和形成疼痛的中枢致敏中起重要作用。Ro 25-6981是近年来备受关注的一种高选择性NR2B拮抗剂。本研究采用大鼠切口痛模型,通过鞘内给药Ro 25-6981预处理,研究瑞芬太尼术后镇痛效果及术后痛感的改变。 方法:采用鞘内注射Ro 25-6981后的足部退足机械阈值和足部退足热潜伏期评价大鼠行为变化。Western blot检测脊髓背角酪氨酸磷酸化NR2B的表达情况。 结果:鞘内注射Ro 25-6981可显著提高术后足部退足机械阈值和足部退足热潜伏期。鞘内注射Ro 25-6981 800.0 μg及术后2h斜拉试验度及BBB评分发生显著变化。Ro 25-6981预处理可降低模型大鼠脊髓背角中酪氨酸磷酸化NR2B的高水平表达。 结论:鞘内注射Ro 25-6981对大鼠切口疼痛有明显的镇痛作用,可有效减轻瑞芬太尼术后引起的痛觉过敏。[3] |
| 动物实验 |
Animal/Disease Models: 6-OHDA injured rat [1]
Doses: 0.39-12.5 mg/kg Route of Administration: intraperitoneal (ip) injection Experimental Results: Dose-dependent induction of opposite tight nasal-caudal rotation and weak co-directional rotation response, indicating Effect of mildly non-specific stimulating compounds. Animal/Disease Models: Male albino rats of Wistar strain [2] Doses: 1, 3 mg/kg Route of Administration: Ip Experimental Results: N1-P2 amplitude was Dramatically diminished at the higher stimulation intensity of 3 mg/kg, and demonstrated Age- and activation-dependent anticonvulsant effects in early postnatal development. Twenty-three months after exposure to MPTP, a group of four animals was treated with either vehicle or one of the three doses of Ro 25-6981 or levodopa in combination with one of two doses of Ro 25-6981. In these experiments, we used a Latin square design for the allocation of treatments. A 1-week recovery period was allowed between experiments. The animals had previously been treated with a selective D1 agonist for 25 days, and with a combination of a monoamine blocker in combination with levodopa/carbidopa for 30 days. Drug-free intervals between the experimental treatments were 9 and 12 months, respectively. These treatment did not lead to the development of dyskinesias. [1] Each age group was formed by control animals treated with saline and two groups treated with different doses of Ro 25-6981. Every age and dose group consisted of eight animals. Ro 25-6981 maleate ((αR, βS)-α-(4-hydroxyphenyl)-β-methyl-4-(phenylmethyl)-1-piperidinepropanol maleate) was freshly dissolved in saline (1 mg/ml) before beginning of each experiment. The drug was administered intraperitoneally in doses of 1 or 3 mg/kg. [2] Single pulse evoked potentials [2] Single 1-ms pulses with intensities increasing from 0.4 to 5.0 mA (0.4, 0.6, 0.8, 1.0, 1.4, 1.8, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5 and 5.0 mA) were applied. First cycle of stimulations was a control one, then Ro 25-6981 or saline were injected and 20 min later the second stimulation series started. The software automatically averaged five subsequent responses (at each of 13 different current intensities used) and the amplitude was measured between peaks of N1 (first negative) and P2 (second positive) waves (Fig. 1a). First positive wave could not be used because it was often distorted by stimulation artifact. Paired pulse evoked potentials [2] The threshold stimulation intensity was found for each animal and double times this intensity was used to elicit paired responses with interpulse intervals from 50 to 1000 ms. Two cycles of stimulations were again performed: first before administration of the drug, and the second 20 min after Ro 25-6981 or saline administration. Amplitude of the first (A1) and second (A2) response was again measured between peaks of N1 (first negative) and P2 (second positive) waves (Fig. 1b). The A2/A1 ratio was calculated for each interval. Cortical afterdischarges (ADs) [2] Series of 1-ms biphasic rectangular pulses were applied at 8-Hz frequency for 15 s (see an example of ADs recording in Fig. 2). Stimulation with suprathreshold current intensity was repeated six times. Intensity of 3.0 mA was reliably suprathreshold in P18 and P25 rats, higher stimulation intensity (up to 5.0 mA) was necessary in P12 animals due to immaturity of neuronal circuits (Mares et al., 2002). Intervals between two stimulation series were 20 min. The Ro 25-6981 or saline were always injected 10 min after the first AD (i.e. after the predrug control stimulation). Additional series with 30-min intervals were used in 12-day-old rats to determine the duration of effects of Ro 25-6981. Ro 25-6981 was dissolved in DMSO (dimethyl sulfoxide, 5%) to a volume of 25 μl. Remifentanil (0.04 mg/kg) was dissolved in saline (NaCl 0.9%) to a volume of 0.4 ml. Intrathecal injection of Ro 25-6981 was performed at 30 min prior plantar incision. Remifentanil (0.04 mg/kg, 0.4 ml) was infused subcutaneously over a period of 30 min using an apparatus pump. The infusion rate was 0.8 ml/h. Control animals received the same volume of saline under identical conditions. Drug administration and experimental grouping [3] All rats were anesthetized with sevoflurane by a nose mask. Ro 25-6981 was dissolved in 5% DMSO. Remifentanil hydrochloride (0.04 mg/kg) was dissolved in saline (NaCl 0.9%) to a volume of 0.4 ml. According to the different doses administered, 54 SD rats were randomly divided into 9 groups (n = 6) (Table 1). Within two weeks before the experiment, the rats were placed in the test room for 2 h every day to accustom various apparatuses. The drug Ro 25-6981 was injected intrathecally before surgical incision. The detailed information of the dose was shown in Table 1. Intrathecal injections (i.t.) were made through the intervertebral space in all rats between the L4 and L5 of the spinal cord, as described by Hylden and Wilcox (1980). Ro 25-6981 (dissolved in 5% DMSO) at the dose of 25 μl was administrated i.t. with a 28-gauge 1/2-inch stainless steel needle connected to a 50 μl Hamilton microsyringe, the animal being lightly restrained to maintain the position of the needle. Puncture of the dura was indicated behaviorally by a slight flick of the tail. Because intrathecal injection of 5% DMSO solvent had no effect on the rat behavior (Qu et al., 2009), in order to maintain consistency, all the rats received intrathecal injection with 5% DMSO solvent. Rat models of incisional pain in the right back paw were prepared after intrathecal injection in all groups except group C. In group M, (R + M)1, (R + M)2 and (R + M)3, remifentanil (0.04 mg/kg, 0.4 ml) was infused subcutaneously during surgical incision with a pump for 30 min, and in group C, I, R1, R2 and R3, 0.9% saline (0.4 ml) was infused subcutaneously in identical conditions for 30 min. For behavioral studies, paw withdrawal mechanical threshold (PWMT) and paw withdrawal thermal latency (PWTL) of the rats were tested. The changes of rat behavior were measured at 24 h before intrathecal injection and at 2 h, 6 h, 24 h, and 48 h after operation (n = 6). And the motor function indexes (inclined pull test and BASSO, BEATTIE and BRESNAHAN (BBB) rating) were also examined at the same time points. According to the changes in behavioral indicators of pain, the specimens of all groups were collected at 2 h, 6 h, and 48 h after operation (n = 4) for Western blot analysis. |
| 参考文献 |
|
| 其他信息 |
Ro 25-6981 is a member of the class of piperidines that is 4-benzylpiperidine substituted by a 3-hydroxy-3-(4-hydroxyphenyl)-2-methylpropyl group at position 1 (the 1R,2S-stereoisomer). It is a potent antagonist of the GluN2B subunit of the N-methyl-D-aspartate (NMDA) receptor. It has a role as a NMDA receptor antagonist, an anticonvulsant, an antidepressant and a neuroprotective agent. It is a member of piperidines, a member of phenols, a secondary alcohol, a tertiary amino compound and a member of benzenes. It is a conjugate base of a Ro 25-6981(1+).
Ro 25-6981, an activity-dependent antagonist of NMDA receptors containing the NR2B subunit, had antiparkinsonian activity in two relevant animal models of PD. Ro 25-6981-induced contraversive rotations in 6-OHDA-lesioned rats without stimulating locomotor activity in normal rats. In addition, Ro 25-6981 increased locomotor activity and reduced disability in MPTP-treated marmosets. More importantly, Ro 25-6981 potentiated the action of levodopa in both models and increased the efficacy of apomorphine and a D1- and D2-selective dopamine receptor agonist in 6-OHDA-lesioned rats. Furthermore, Ro 25-6981 attenuated the peak levodopa response in 6-OHDA-lesioned rats chronically treated with levodopa without reducing the overall response. Ro 25-6981 did not lead to any obvious motor impairment. Thus, the antiparkinsonian action of Ro 25-6981 is superior to that of any conventional competitive and non-competitive NMDA receptor antagonist in that it combines intrinsic antiparkinsonian activity with the potency to synergistically interact with levodopa and both D1 and D2 receptor agonists. In contrast, conventional NMDA receptor antagonists do not have antiparkinsonian actions in the absence of dopaminergic stimulation and selectively interact with subtype-specific agonists Klockgether and Turski, 1990, Löschmann et al., 1997, Morelli et al., 1992. There are numerous reasons to assume that the striatum mediates the antiparkinsonian action of Ro 25-6981. In situ hybridisation studies show that NR2B subunits are expressed in the striatum at higher levels than in other basal ganglia nuclei (Kosinski et al., 1998). Accordingly, binding of [3H]Ro 25-6981 was found to be high in the rat striatum compared to the external pallidum (Fischer et al., 1997). Furthermore, local intrastriatal injection of NMDA produces parkinsonism in rats, while intrastriatal injection of ifenprodil, another antagonist with preference for NR2B-containing NMDA receptors, reversed parkinsonism in 6-OHDA-lesioned marmosets Klockgether and Turski, 1993, Mitchell et al., 1995. The current model of the pathogenesis of PD proposes that striatal neurons projecting to the external pallidum (indirect pathway) become overactive following dopamine depletion, while the activity of striatal neurons projecting directly to the basal ganglia output nuclei (direct pathway) is reduced (Albin et al., 1989). Since NR2B receptors are located on striatal projection neurons Landwehrmeyer et al., 1995, Standaert et al., 1999, it seems likely that the effects of Ro 25-6981 are the result of blockade of NR2B-containing NMDA receptors on striatal neurons projecting to the external pallidum. This assumption implies a selective suppressive action of Ro 25-6981 on the indirect pathway without major actions on the (underactive) direct pathway. This selectivity is best explained by the activity-dependance of the actions of Ro 25-6981. Ro 25-6981 only binds with high affinity to activated receptors, while it has negligible effects on non-activated receptors (Fischer et al., 1997). Indeed, the ability of ifenprodil, a structural analogue of Ro 25-6981, to inhibit binding of the NMDA channel blocker MK-801 in the striatum was shown to increase by a factor of four following dopamine depletion (Nash et al., 1999). Ro 25-6981 potentiated the antiparkinsonian action of both, the D1 receptor agonist A68930 and the D2 receptor agonist quinpirole. In contrast, MK-801 potentiated rotational behaviour induced by the D1 receptor agonist SKF 38393, but reduced quinpirole-induced rotational behaviour (Morelli et al., 1992). CPP, on the other hand, potentiated quinpirole-induced rotational behaviour, but not A68930-induced rotations (Löschmann et al., 1997). We propose that the potentiating action of Ro 25-6981 on quinpirole-induced rotations is due to a synergistic suppressive action of both compounds on the indirect pathway. In contrast, the synergistic interaction of the D1 agonist A68930 and Ro 25-6981 is best explained by a combination of an activation of the direct pathway by A68930 and suppression of the indirect pathway by Ro 25-6981. The usefulness of conventional NMDA antagonists as antiparkinsonian drugs is limited by their liability to produce incoordination and ataxia which is due to an action of these compounds on NMDA receptors in the cerebellum (Löscher and Honack, 1991). Since expression of NR2B subunits is low or absent in the cerebellum (Standaert et al., 1994), NR2B-selective NMDA receptor antagonists are predicted to be free of the ataxia-inducing side effects of conventional NMDA antagonists. Indeed, in doses up to 100 mg/kg i.p. Ro 25-6981 did not produce motor impairment in mice (Boyce et al., 1999). This dose is approximately one order of magnitude higher than the dose required to produce an antiparkinsonian action. The failure of Ro 25-6981 to induce ataxia and motor impairments in rodents and primates may therefore contribute to persistent antiparkinsonian activity of Ro 25-6981 even at high doses. Other antagonists with preference for NR2B-containing NMDA receptors had similarly promising actions in animal models of PD. Ifenprodil stimulated locomotor activity in reserpine-treated rats, bilaterally 6-OHDA-lesioned marmosets and MPTP-treated marmosets Mitchell et al., 1995, Nash et al., 1999, Nash et al., 2000. However, a small clinical trial in PD patients was negative (Montastruc et al., 1992). Another NR2B antagonist, CP 101,106, reduced rigidity and akinesia in MPTP-treated non-human primates and haloperidol-treated rats (Steece-Collier et al., 2000). The observation that several structurally unrelated antagonists that share a preference for NMDA receptors containing the NR2B subunit have potent antiparkinsonian actions makes it unlikely that the action of Ro 25-6981 is due to actions at other receptors, in particular dopamine receptors. Indeed, binding experiments revealed only negligible affinity to dopamine receptors (Mutel et al., 1998). A dopamine releasing action of Ro 25-6981 is unlikely in view of the contraversive rotations induced by this compound. While the ability of compounds to elicit contraversive rotations of 6-OHDA-lesioned rats is generally considered to reflect an antiparkinsonian activity (Kaakkola and Teravainen, 1990), numerous observations show that repeated and intermittent administration of levodopa results in a behavioural sensitization with an increased and shortened response that serves as a model of dyskinesias and the wearing-off phenomenon occurring in PD patients after long-term levodopa treatment (Papa et al., 1994). Although these rats do not develop overt dyskinesias, the response alterations occurring after chronic levodopa treatment are reminiscent of response fluctuations in patients with advanced PD. The validity of this model is supported by its ability to predict the antidyskinetic effect of the NMDA antagonist amantadine Papa et al., 1995, Verhagen et al., 1998. Since the plastic changes of the levodopa response in 6-OHDA-lesioned rats are causally associated with an abnormal phosphorylation of striatal NR2B subunits, we were interested to investigate the action of Ro 25-6981 in this model. Chronic levodopa treatment, as performed in this series of experiments, led to an increased peak, but not to a shortened rotational response. This discrepancy to the results published by Papa et al. (1994) may be because of the fact that we did not use the methylester of levodopa. In addition, we used a more liberal selection procedure with a higher dose of apomorphine (0.1 vs. 0.05 mg/kg) that may have resulted in inclusion of rats with only partial 6-OHDA lesions (Papa et al., 1994). Ro 25-6981 reduced the peak rotational response in chronically levodopa-treated 6-OHDA-lesioned rats suggesting a beneficial action of Ro 25-6981 on levodopa-induced dyskinesias and response fluctuations. On the basis of the present results, it appears that selective antagonism at NR2B subunit containing NMDA receptors is sufficient to induce antiparkinsonian effects in two relevant animal models of PD. NR2B antagonists such as Ro 25-6981 could have favourable effects in PD patients and may be used alone or in combination with standard dopaminergic drugs in the management of PD. [1] It seems possible, that after stimulation with increasing intensities the autoreceptor function of presynaptic NR2B/NMDARs in P25 animals can be restored, as it was observed in epileptic adults (Yang et al., 2006). Hence, modulated by the NR2B/NMDARs, GABA release activity, which probably was brought back by high intensity stimulation, might not be blocked completely by the lower (1-mg/kg) dose of Ro 25-6981; therefore, it is possible that it caused the decrease in amplitude of single evoked potentials. Moreover, as in adult animals NR2B subunit is primarily expressed in the forebrain (Loftis and Janowsky, 2003), it is also likely that purely cortical activity can be affected by Ro 25-6981 even in the third and fourth postnatal week in rats. Unfortunately, at the moment we have no explanation on the complete lack of effects of Ro 25-6981 on cortical potentiation or depression induced by paired pulse stimulation. The reason might be in relatively moderate intensity of stimulation (twofold threshold); in contrast, the effects on single-pulse responses were observed mostly at high stimulation intensities. In conclusion, our results show that Ro 25-6981, a selective antagonist of NR2B/NMDARs, exhibits clear activation-dependent anticonvulsant action against epileptic afterdischarges (model of myoclonic seizures) only during the second postnatal week in rats. Consequently, we can imply that in older animals, receptors containing NR2B subunit do not contribute to generation of seizures in this model. Thus, Ro 25-6981 or other NR2B antagonists may represent a useful tool in pharmacotherapy of epileptic activity in immature brain. This drug also significantly reduces the level of physiological excitability induced by single pulse stimulation of sensorimotor cortex in an age-independent manner and did not affect cortical excitability evoked by paired pulse stimulation. At the moment we cannot fully explain this in vivo action of Ro 25-6981 and further tests will be necessary. However, the level of activation of NMDARs containing NR2B subunit and their localization may play a role in the effects of Ro 25-6981 on cortical excitability. [2] NR2B subunit in the generation of pain and central sensitization plays an important role (LoGrasso and McKelvy, 2003). There are seven tyrosine phosphorylation sites in the cytoplasmic C-terminal of NR2B subunit and Tyr-1472 is the main site. Its phosphorylation has significant effect for changes of synaptic plasticity and occurrence of long-term potentiation (LTP). LTP and OIH have the common pharmacological and signal transduction pathway (Drdla et al., 2009, Nakazawa et al., 2001). Many studies have shown that tyrosine phosphorylation of the NR2B at Tyr-1472 in the spinal dorsal horn contributed to the development of hyperalgesia in neuropathic pain model (Abe et al., 2005) and inflammatory pain model (Guo et al., 2002). Therefore, we not only observed the behavioral changes in rats but also Ro 25-6981 induced antinociception in the rat model of incisional pain and hyperalgesia. Western blot analysis showed that pre-intrathecal injection of Ro 25-6981 decreased the higher level expression of tyrosine phosphorylation of NR2B in spinal dorsal horn and hyperalgesia. Ro 25-6981 may relieve incisional pain and remifentanil-induced hyperalgesia through the pathway of NR2B as the specific NR2B subunit antagonist. In summary, behavioral test and Western blot analysis demonstrated pre-intrathecal injection of Ro 25-6981 had significant analgesic effects on incision pain in rats and effectively prevented postoperative hyperalgesia induced by remifentanil. These results might have some relation with inhibiting tyrosine phosphorylation of NR2B in superficial spinal cord of rats. The effects of Ro 25-6981 on locomotor functions of rats changed with the different doses and drug metabolism. Although Ro 25-6981 is currently studied for animal experiment, clinical application has not been carried out. The mechanism of these drugs may provide ideas to find out more value methods for the clinical treatment of incisional pain and hyperalgesia. Conclusively, our study investigated the analgesic effects through targeting NR2B in a rat incision pain model. And our data suggest that targeting NR2B in the spinal cord might be a new strategy for the treatment of clinical pain. [3] |
| 分子式 |
C22H29NO2
|
|---|---|
| 分子量 |
339.479
|
| 精确质量 |
339.219
|
| 元素分析 |
C, 77.84; H, 8.61; N, 4.13; O, 9.43
|
| CAS号 |
169274-78-6
|
| 相关CAS号 |
Ro 25-6981 Maleate;1312991-76-6;Ro 25-6981 hydrochloride;919289-58-0
|
| PubChem CID |
6604887
|
| 外观&性状 |
White to off-white solid powder
|
| 沸点 |
703.7ºC at 760mmHg
|
| 闪点 |
379.4ºC
|
| 蒸汽压 |
2.52E-11mmHg at 25°C
|
| 折射率 |
1.587
|
| LogP |
3.666
|
| tPSA |
118.3
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
25
|
| 分子复杂度/Complexity |
366
|
| 定义原子立体中心数目 |
2
|
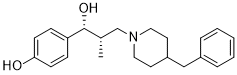
| SMILES |
C[C@@H](CN1CCC(CC1)CC2=CC=CC=C2)[C@H](C3=CC=C(C=C3)O)O
|
| InChi Key |
WVZSEUPGUDIELE-HTAPYJJXSA-N
|
| InChi Code |
InChI=1S/C22H29NO2/c1-17(22(25)20-7-9-21(24)10-8-20)16-23-13-11-19(12-14-23)15-18-5-3-2-4-6-18/h2-10,17,19,22,24-25H,11-16H2,1H3/t17-,22+/m0/s1
|
| 化学名 |
4-((1R,2S)-3-(4-benzylpiperidin-1-yl)-1-hydroxy-2-methylpropyl)phenol
|
| 别名 |
Ro 25-6981; Ro25-6981; Ro-25-6981; Ro-256981; 169274-78-6; Ro 25-6981; Ro-25-6981; ro25-6981; Ro 25-6981 free base; CHEBI:92897; (alphar,betas)-alpha-(4-hydroxyphenyl)-beta-methyl-4-(phenylmethyl)-1-piperidinepropanol; 4-[(1R,2S)-1-hydroxy-2-methyl-3-[4-(phenylmethyl)-1-piperidinyl]propyl]phenol; Ro 256981; Ro256981; Ro 25-6981 free base
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9457 mL | 14.7284 mL | 29.4568 mL | |
| 5 mM | 0.5891 mL | 2.9457 mL | 5.8914 mL | |
| 10 mM | 0.2946 mL | 1.4728 mL | 2.9457 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。