| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
Topo I (DU-145 Luc cells) ( IC50 = 2 nM ); Topo I (MCF-7 Luc cells) ( IC50 = 13 nM )
|
|---|---|
| 体外研究 (In Vitro) |
托泊替康盐酸盐水合物以剂量和时间依赖性方式强烈抑制人胶质瘤细胞和胶质瘤干细胞 (GSC) 的生长 [1]。与对照组相比,托泊替康盐酸盐水合物 (0–40 μM) 以剂量依赖性方式显着降低细胞活力 [1]。盐酸托泊替康水合物对 U251、U87、GSCs-U251 和 GSCs-U87 细胞表现出抗增殖活性,IC50 值分别为 2.73±0.25、2.95±0.23、5.46±0.41 和 5.95±0.24 μM [1]。
|
| 体内研究 (In Vivo) |
盐酸托泊替康水合物(0.5、1.0 和 1.5 mg/kg;口服,每日)导致卵巢癌模型中微血管密度大幅下降,而每日口服托泊替康 1.5 mg/kg 治疗的患者大鼠的食物摄入量较低,抗肿瘤效果较差[2]。
|
| 酶活实验 |
拓扑替康[(S)-9-二甲氨基甲基-10-羟基喜树碱盐酸盐;SK&F 104864-A,NSC 609699]是喜树碱的水溶性半合成类似物,是一种强效的拓扑异构酶I抑制剂。在这里,我们表明拓扑替康稳定了抗辐射的人类B系急性淋巴细胞白血病(ALL)细胞中的拓扑异构酶I/DNA可切割复合物,尽管bcl-2蛋白表达水平很高,但仍会导致快速凋亡细胞死亡,并以剂量依赖的方式抑制ALL细胞的体外克隆生长。此外,拓扑替康在三种不同的人类预后不良ALL的严重联合免疫缺陷(SCID)小鼠模型中引发了强效的抗白血病活性,并显著提高了SCID小鼠在全身药物暴露水平下用致命剂量的人类白血病细胞攻击的无事件生存率,这在白血病儿童中很容易实现[Blood. 1995 May 15;85(10):2817-28]。
|
| 细胞实验 |
胶质瘤是最恶性的脑肿瘤,含有一小部分胶质瘤干细胞(GSCs),与治疗耐药性和肿瘤复发有关。拓扑异构酶I抑制剂紫草素和拓扑替康在抗癌治疗中发挥着至关重要的作用。成功分离和鉴定胶质瘤细胞中的GSCs后,在不同时间点用不同浓度的紫草素或拓扑替康给药U251、U87、GSCs-U251和GSCs-U87细胞,以寻求最佳给药浓度和时间点。采用细胞计数试剂盒-8和流式细胞仪检测细胞活力、细胞周期和凋亡,观察对胶质瘤细胞和GSCs的抑制作用。我们证明,紫草素和拓扑替康不仅明显抑制人脑胶质瘤细胞的增殖,而且以剂量和时间依赖的方式抑制GSCs的增殖。根据24小时的IC50值,选择2μmol/L的紫草素和3μmol/L的拓扑替康作为最佳给药浓度。此外,紫草素和拓扑替康诱导细胞周期阻滞在G0/G1期和S期,并促进细胞凋亡。Bcl-2表达的下调与胱天蛋白酶9/3依赖途径的激活有关。因此,上述结果表明,拓扑异构酶I抑制剂紫草素和拓扑替康抑制了GSCs和胶质瘤细胞的生长并诱导了它们的凋亡,这表明它们可能是靶向胶质瘤的潜在抗癌药物,从而提供了一种新的治疗策略[1]。
|
| 动物实验 |
In vivo antitumor efficacies of the LDM topotecan and pazopanib as single agents and in combination were tested on 4 subcutaneous xenograft models and on 2 neuroblastoma metastatic models. Circulating angiogenic factors such as circulating endothelial cells (CEC), circulating endothelial pro genitor cells (CEP), and microvessel densities were used as surrogate biomarker markers of antiangiogenic activity.[2]
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Renal clearance is an important determinant of topotecan elimination. In a mass balance/excretion study in 4 patients with solid tumors, the overall recovery of total topotecan and its N-desmethyl metabolite in urine and feces over 9 days averaged 73.4 ± 2.3% of the administered IV dose. Fecal elimination of total topotecan accounted for 9 ± 3.6% while fecal elimination of N-desmethyl topotecan was 1.7 ± 0.6%. The pharmacokinetics of topotecan have been extensively studied in patients with normal renal function and there is one study of patients with mild to moderate renal insufficiency. However, the effect of hemodialysis on topotecan disposition has not been reported. The objective of this study was to characterize the disposition of topotecan in a patient with severe renal insufficiency receiving hemodialysis. Topotecan lactone disposition was characterized in a patient on and off hemodialysis. The topotecan lactone clearance determined after administration of topotecan alone and with hemodialysis was 5.3 L/hr per sq m vs 20.1 L/hr per sq m respectively. At 30 min after the completion of hemodialysis, the topotecan plasma concentration obtained was greater than that measured at the end of hemodialysis (i.e. 8.0 ng/mL vs 4.9 ng/mL), suggesting a rebound effect. The topotecan terminal half-life off dialysis was 13.6 hr, compared with an apparent half-life determined during hemodialysis of 3.0 hr. These results demonstrate that topotecan plasma clearance while on hemodialysis increased approximately fourfold. Hemodialysis may be an effective systemic clearance process for topotecan and should be considered in selected clinical situations (e.g. inadvertent overdose, severe renal dysfunction). In lactating rats receiving IV topotecan at a dosage of 4.72 mg/sq m, high concentrations of the drug (i.e., up to 48 times higher than plasma concentrations) were distributed into milk. It is not known whether topotecan is distributed into human milk. Following oral administration, about 57% of topotecan (administered daily for 5 days) is excreted in urine as unchanged drug (20%) and as the N-desmethyl metabolite (2%).47 Approximately 33% of the oral dose of topotecan was eliminated in feces as total topotecan and approximately 2% as N-desmethyl topotecan. Following IV administration, about 74% of a topotecan dose is excreted, mostly unchanged in urine (51%) and feces (18%) within 9 days; excretion of N-desmethyl topotecan in urine is approximately 3% and in feces is approximately 2%. O-Glucuronide metabolites of topotecan and N-desmethyl topotecan also have been detected in urine following oral and IV (less than 2% of the administered IV dose) administration of the drug. No substantial gender-related differences in pharmacokinetics were reported in patients receiving oral topotecan. The average plasma clearance of IV topotecan was 24% higher in males than in females, mainly because of difference in body size. For more Absorption, Distribution and Excretion (Complete) data for Topotecan (6 total), please visit the HSDB record page. Metabolism / Metabolites Topotecan undergoes a reversible pH dependent hydrolysis of its lactone moiety; it is the lactone form that is pharmacologically active. Topotecan undergoes a reversible pH-dependent hydrolysis of its lactone moiety; it is the lactone form that is pharmacologically active. At pH =4, the lactone is exclusively present, whereas the ring-opened hydroxy-acid form predominates at physiologic pH. In vitro studies in human liver microsomes indicate topotecan is metabolized to an N-demethylated metabolite. The mean metabolite:parent AUC ratio was about 3% for total topotecan and topotecan lactone following IV administration. Biological Half-Life 2-3 hours The pharmacokinetics of topotecan have been evaluated in cancer patients following doses of 0.5 to 1.5 mg/sq m administered as a 30-minute infusion. Topotecan exhibits multiexponential pharmacokinetics with a terminal half-life of 2 to 3 hours. Topotecan has a terminal half-life of 3-6 hours following oral administration and 2-3 hours following IV administration of the drug. ... The objective of this study was to characterize the disposition of topotecan in a patient with severe renal insufficiency receiving hemodialysis. ... The topotecan terminal half-life off dialysis was 13.6 hr, compared with an apparent half-life determined during hemodialysis of 3.0 hr. ... |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Most sources consider breastfeeding to be contraindicated during maternal high-dose antineoplastic drug therapy. The manufacturer recommends that women not breastfeed during treatment with topotecan and for 1 week after the last dose. Chemotherapy may adversely affect the normal microbiome and chemical makeup of breastmilk. Women who receive chemotherapy during pregnancy are more likely to have difficulty nursing their infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 35% |
| 参考文献 |
|
| 其他信息 |
Gliomas, the most malignant form of brain tumors, contain a small subpopulation of glioma stem cells (GSCs) that are implicated in therapeutic resistance and tumor recurrence. Topoisomerase I inhibitors, shikonin and topotecan, play a crucial role in anti-cancer therapies. After isolated and identified the GSCs from glioma cells successfully, U251, U87, GSCs-U251 and GSCs-U87 cells were administrated with various concentrations of shikonin or topotecan at different time points to seek for the optimal administration concentration and time point. The cell viability, cell cycle and apoptosis were detected using cell counting kit-8 and flow cytometer to observe the inhibitory effects on glioma cells and GSCs. We demonstrated that shikonin and topotecan obviously inhibited proliferation of not only human glioma cells but also GSCs in a dose- and time-dependent manner. According to the IC50 values at 24 h, 2 μmol/L of shikonin and 3 μmol/L of topotecan were selected as the optimal administration concentration. In addition, shikonin and topotecan induced cell cycle arrest in G0/G1 and S phases and promoted apoptosis. The down-regulation of Bcl-2 expression with the activation of caspase 9/3-dependent pathway was involved in the apoptosis process. Therefore, the above results showed that topoisomerase I inhibitors, shikonin and topotecan, inhibited growth and induced apoptosis of GSCs as well as glioma cells, which suggested that they might be the potential anticancer agents targeting gliomas to provide a novel therapeutic strategy.[1]
Purpose: Low dose metronomic (LDM) chemotherapy, combined with VEGF signaling pathway inhibitors, is a highly effective strategy to coordinately inhibit angiogenesis and tumor growth in many adult preclinical cancer models. We have tested the efficacies of daily oral LDM topotecan alone and in combination with pazopanib, a VEGF receptor inhibitor, in three pediatric extracranial solid tumor mouse models. Experimental design: In vitro dose-response study of topotecan and pazopanib was conducted on several neuroblastoma, osteosarcoma, and rhabdomyosarcoma cell lines. In vivo antitumor efficacies of the LDM topotecan and pazopanib as single agents and in combination were tested on 4 subcutaneous xenograft models and on 2 neuroblastoma metastatic models. Circulating angiogenic factors such as circulating endothelial cells (CEC), circulating endothelial pro genitor cells (CEP), and microvessel densities were used as surrogate biomarker markers of antiangiogenic activity. Results: In vitro, topotecan caused a dose-dependent decrease in viabilities of all cell lines, while pazopanib did not. In vivo, combination of topotecan + pazopanib (TP + PZ) showed significant antitumor activity and significant enhancement in survival compared with the respective single agents in all models. Reductions in viable CEP and/or CEC levels and tumor microvessel density were correlated with tumor response and therefore confirmed the antiangiogenic activity of the regimens. Pharmacokinetic studies of both drugs did not reveal any drug-drug interaction. Conclusion: Metronomic administration of TP + PZ showed a statistically significant antitumor activity compared with respective single agents in pediatric tumor mouse models and represent a valid option as a maintenance therapy in aggressive pediatric solid tumors.[2] |
| 分子量 |
475.922045230865
|
|---|---|
| 精确质量 |
475.151
|
| CAS号 |
1044663-62-8
|
| 相关CAS号 |
Topotecan hydrochloride;119413-54-6; 123948-87-8; 1044663-62-8 (Topotecan HCl hydrate)
|
| PubChem CID |
73440824
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| tPSA |
104Ų
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
867
|
| 定义原子立体中心数目 |
1
|
| SMILES |
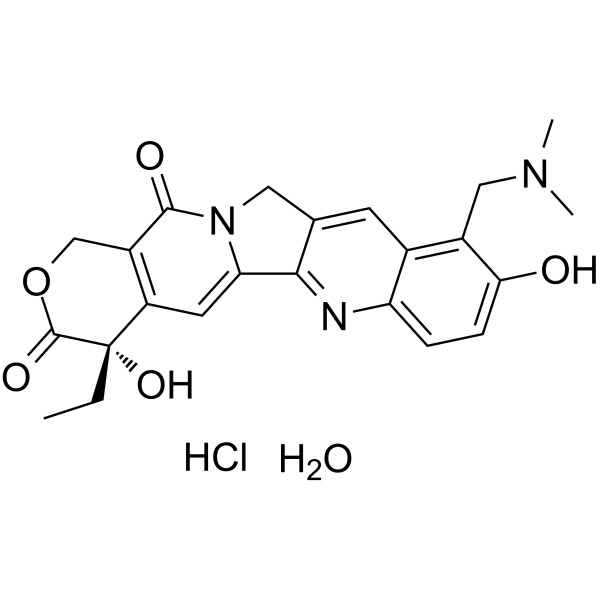
CC[C@@]1(C2=C(COC1=O)C(=O)N3CC4=CC5=C(C=CC(=C5CN(C)C)O)N=C4C3=C2)O.O.Cl
|
| InChi Key |
XVYBIGDRQOMVJX-IFUPQEAVSA-N
|
| InChi Code |
InChI=1S/C23H23N3O5.ClH.H2O/c1-4-23(30)16-8-18-20-12(9-26(18)21(28)15(16)11-31-22(23)29)7-13-14(10-25(2)3)19(27)6-5-17(13)24-20;;/h5-8,27,30H,4,9-11H2,1-3H3;1H;1H2/t23-;;/m0../s1
|
| 化学名 |
(19S)-8-[(dimethylamino)methyl]-19-ethyl-7,19-dihydroxy-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4(9),5,7,10,15(20)-heptaene-14,18-dione;hydrate;hydrochloride
|
| 别名 |
Topotecan hydrochloride hydrate; Topotecan (hydrochloride hydrate); 1044663-62-8; SCHEMBL13731135; Topotecan hydrochloride hydrate; Topotecan (hydrochloride hydrate); 1044663-62-8; SCHEMBL13731135; Tox21_500905; NCGC00261590-01; Tox21_500905; CCG-222209; NCGC00261590-01;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1012 mL | 10.5060 mL | 21.0119 mL | |
| 5 mM | 0.4202 mL | 2.1012 mL | 4.2024 mL | |
| 10 mM | 0.2101 mL | 1.0506 mL | 2.1012 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02030964 | Active Recruiting |
Drug: Topotecan Drug: DFMO |
Neuroblastoma | New Approaches to Neuroblastoma Therapy Consortium |
December 2013 | Phase 1 |
| NCT02298348 | Active Recruiting |
Drug: Topotecan Drug: Sorafenib |
Neuroblastoma | New Approaches to Neuroblastoma Therapy Consortium |
April 2015 | Phase 1 |
| NCT03600649 | Active Recruiting |
Drug: Topotecan Drug: Seclidemstat |
Ewing Sarcoma Myoepithelial Tumor |
Salarius Pharmaceuticals, LLC | June 4, 2018 | Phase 1 |
| NCT02487095 | Active Recruiting |
Drug: Topotecan Drug: VX-970 (M6620) |
Small Cell Lung Carcinoma Ovarian Neoplasms |
National Cancer Institute (NCI) |
July 30, 2015 | Phase 1 Phase 2 |
| NCT00638898 | Active Recruiting |
Drug: topotecan hydrochloride Drug: busulfan |
Solid Tumor Ewing Sarcoma |
City of Hope Medical Center | February 26, 2007 | Phase 1 |