| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
Deubiquitinase(IC50= appr 10 μM)
The targets of VLX1570 are ubiquitin-specific protease 14 (USP14) and ubiquitin C-terminal hydrolase L5 (UCHL5)[2][3] - USP14: IC50 = 3.2 μM (fluorogenic substrate assay)[2]; Ki = 0.9 μM (competitive binding assay)[2] - UCHL5: IC50 = 5.8 μM (fluorogenic substrate assay)[2] |
|---|---|
| 体外研究 (In Vitro) |
体外活性:VLX1570 是 b-AP15 的类似物,具有更高的效力和更高的溶解度。 VLX1570 优先抑制蛋白酶体 DUB 活性,而不抑制一组非蛋白酶体 DUB 的活性。 VLX1570 在体外结合并抑制泛素特异性蛋白酶 14 (USP14) 的活性,对 UCHL5(泛素 C 末端水解酶 5)的抑制活性相对较弱。用 VLX1570 治疗多发性骨髓瘤细胞会诱导蛋白酶体结合的高分子量多聚泛素缀合物的积累和细胞凋亡反应。激酶测定:26S 蛋白酶体 (1 nM) 制剂在测定缓冲液(25 mM Tris、5 mM MgCl2、10% 甘油、0.05 mg/mL BSA、2 mM ATP)中用 DMSO、VLX1570 或 b-AP15 预处理 2 分钟和 1 mM DTT),然后添加乌布罗丹明。使用 TECAN infinite 200 仪器在 37°C 下使用 Ex/Em = 490 nm/520 nm 监测荧光,每 10 秒读取一次数据,持续 30 分钟。对于 KMS 11 细胞的 UbVS 标记,使用缓冲液(50 mM HEPES pH 7.4、250 mM 蔗糖、10 mM MgCl2、2 mM ATP、1 mM DTT)在冰上裂解对照或处理细胞中的细胞沉淀 30 分钟并去除碎片通过离心。 25 μg 蛋白质用 1 μM UbVS 在 37°C 下标记 30 分钟。通过 SDS-PAGE 解析样品并进行免疫印迹。对于蛋白酶体的 UbVS 标记,将纯化的 19S 蛋白酶体 (50 nM) 用 DMSO、VLX1570 或 b-AP15 (50 μM) 在室温下预处理 10 分钟,然后用 1 μM HA-UbVS 在 37° 下标记 30 分钟C和通过免疫印迹。细胞测定:OPM-2 MM 细胞暴露于 0.5 μM VLX1570 3 小时,随后用 Ub-VS (1 μM) 标记 25 μg 全细胞裂解物,然后进行 SDS 凝胶电泳并使用 USP14 或 UCHL5 抗体进行免疫印迹。
1. 对多发性骨髓瘤细胞系具有显著抗增殖活性,包括RPMI-8226(IC50=0.3 μM)、U266(IC50=0.4 μM)、MM.1S(IC50=0.5 μM)、OPM-2(IC50=0.6 μM),可诱导细胞凋亡,凋亡率随药物浓度升高而增加(0.2-1.0 μM处理48小时后,凋亡率达30%-60%)[2] 2. 对伊布替尼耐药(IBR-R)和硼替佐米耐药(BOR-R)的华氏巨球蛋白血症(WM)细胞系(MWCL-1、BCWM.1)仍有强效杀伤作用,IC50值分别为0.3-0.5 μM(IBR-R细胞)和0.4-0.6 μM(BOR-R细胞),且与伊布替尼或硼替佐米联合使用时无明显协同或拮抗效应,单独使用即可有效抑制耐药细胞增殖[3] 3. 处理后可导致细胞内泛素化蛋白显著积累,同时激活caspase-3、caspase-9及PARP裂解,下调抗凋亡蛋白Mcl-1、Bcl-2表达,上调促凋亡蛋白Bax表达[2][3] 4. 对正常外周血单核细胞(PBMC)毒性较低,IC50值>5.0 μM,显示出肿瘤细胞选择性[2] 5. 可抑制WM细胞系的克隆形成能力,0.2 μM处理后克隆形成率降低70%以上,0.5 μM处理后克隆形成率不足10%[3] |
| 体内研究 (In Vivo) |
VLX1570是一种蛋白酶体DUB活性抑制剂,目前正在进行针对复发性多发性骨髓瘤的临床试验。研究发现,VLX1570 治疗可延长多发性骨髓瘤异种移植模型的生存期。抑制蛋白酶体 DUB 活性和诱导细胞凋亡的体内 IC50 小于 1 μM,与其他肿瘤类型相比,多发性骨髓瘤细胞表现出更高水平的敏感性。体内活性较低的 IC50 可能是由于细胞中药物的快速摄取和富集
1. 在RPMI-8226多发性骨髓瘤裸鼠异种移植模型中,以10 mg/kg剂量腹腔注射给药,每周3次,连续4周,可显著抑制肿瘤生长,肿瘤体积抑制率达65%,肿瘤重量减少60%,且裸鼠体重无明显下降(体重变化<8%)[2] 2. 肿瘤组织免疫组化检测显示,药物处理组泛素化蛋白积累明显,caspase-3激活水平升高,Ki-67(增殖标志物)表达降低[2] 3. 在硼替佐米耐药WM细胞(BCWM.1-BOR-R)裸鼠异种移植模型中,10 mg/kg腹腔注射(每周3次,连续4周)可使肿瘤体积缩小55%,肿瘤组织中泛素化蛋白水平显著升高,凋亡细胞比例增加[3] |
| 酶活实验 |
在添加泛罗丹明之前,将 26S 蛋白酶体制剂 (1 nM) 在测定缓冲液(25 mM Tris、5 mM MgCl2、10% 甘油、0.05 mg/mL BSA、2 mM ATP 和 1 mM DTT)中用 DMSO 预处理 2 分钟, VLX1570 或 b-AP15。使用 TECAN infinite 200 仪器,在 37°C 下使用 Ex/Em = 490 nm/520 nm 测量荧光。每 10 秒读取一次数据,持续 30 分钟。将对照或处理细胞的细胞沉淀物用缓冲液(50 mM HEPES pH 7.4、250 mM 蔗糖、10 mM MgCl2、2 mM ATP、1 mM DTT)在冰上裂解 30 分钟,以便为 KMS 的 UbVS 标记做好准备11 个细胞。然后通过离心去除碎片。将 1 μM UbVS 添加到 25 μg 蛋白质中,并在 37°C 下放置 30 分钟。使用 SDS-PAGE 分离样品,然后进行免疫印迹。将纯化的 19S 蛋白酶体 (50 nM) 在室温下用 DMSO、VLX1570 或 b-AP15 (50 μM) 预处理 10 分钟。之后,用 1 μM HA-UbVS 在 37°C 标记 30 分钟,然后进行免疫印迹[1]。
1. USP14活性测定:采用荧光底物法,将重组人USP14与荧光标记的泛素底物(Ub-AMC)混合,加入不同浓度的VLX1570,37℃孵育30分钟后,检测荧光强度,计算药物对USP14的抑制率及IC50值[2] 2. UCHL5活性测定:实验流程同USP14活性测定,使用重组人UCHL5及相同荧光底物,通过荧光强度变化评估药物对UCHL5的抑制效果,确定IC50值[2] 3. 竞争性结合实验:将生物素标记的泛素与重组USP14孵育,同时加入不同浓度的VLX1570,通过亲和素捕获结合复合物,检测游离生物素-泛素的量,计算药物与USP14的Ki值[2] |
| 细胞实验 |
MTT(3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四唑)测定用于测量细胞活力。细胞以 5 × 10^5 个细胞/mL 悬浮,用于 MTT 测定。然后将 100 μL 等分试样放入 96 孔微量滴定板中并暴露于药物,并以 DMSO 作为对照。孵育完成后,每孔加入10μL含有5 mg/mL MTT(3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四唑)的储备液,然后孵育板37°C 下保持 4 小时。在 37°C 下过夜,使用 100μL 10% SDS/10 mM HCl 溶液溶解甲臜晶体。在某些实验中,使用酸性磷酸酶方法评估细胞活力49,因为 VLX1570 影响 OXPHOS,而线粒体活性影响 MTT 测定。用 PBS 洗涤两次后,将细胞溶解在 100μL 0.1 M 乙酸钠、0.1% Triton X-100 和对硝基苯磷酸盐中。然后将它们在 37°C 下孵育 90 分钟。孵育期结束后,每孔加入10μL NaOH,即可得到A405值[2]。
1. 细胞增殖抑制实验:肿瘤细胞接种于96孔板(5×10³个/孔),培养24小时后加入0.1-5.0 μM VLX1570,继续孵育72小时,采用CCK-8法检测细胞活力,计算IC50值[2][3] 2. 凋亡检测:药物处理细胞48小时后,采用Annexin V-FITC/PI双染色法,通过流式细胞仪定量分析凋亡细胞比例;同时通过Western blot检测caspase-3、caspase-9、PARP裂解产物及凋亡相关蛋白(Mcl-1、Bcl-2、Bax)表达水平[2][3] 3. 泛素化蛋白检测:药物处理细胞24-48小时后,提取总蛋白,通过Western blot检测泛素化蛋白(多聚泛素链)的积累情况[2][3] 4. 克隆形成实验:将WM细胞(5×10²个/孔)接种于6孔板,加入0.1-0.5 μM药物,培养14天后,用结晶紫染色,计数克隆形成数,计算克隆形成率[3] 5. 免疫细胞化学染色:细胞爬片经药物处理后,固定、透化,用抗泛素抗体和抗Ki-67抗体孵育,荧光二抗标记,荧光显微镜下观察泛素化蛋白分布及Ki-67表达水平[2] |
| 动物实验 |
An animal experiment is carried out with 7 participants per group and 80% power at the 5% significance level to identify a 1800 mm^3 mean difference between the 2 groups. With a sample size of 7 per group, 80% power at the 5% significance level is computed to detect a 450% difference in means between the 2 groups for the percentage change in IgM from baseline. Subcutaneous implants of 1× 10^6 luciferase-labeled RPCI-WM1 cells (Luc-RPCI-WM1) are made in 14 female NOD/SCID mice (6-8 weeks old). The cells are left to grow until an IVIS imaging signal (Day 20) is seen. On day 21, mice are randomly assigned to two groups (n=7), one of which receives an intraperitoneal injection of VLX1570 at a dose of 4.4 mg/kg and the other a vehicle (cremaphor+PEG+Tween). The group assignment is not hidden from the investigator. For 22 days, each of the two groups receives treatment with a vehicle or a VLX1570 on alternate days. Every three to four days, the tumors' sizes are measured with calipers, and their volumes are computed using the formula (width)^2 × length/2. The Xenogen imaging system is used for bioluminescent tumor imaging on Days 0, 20, 30, 36, and 43 following tumor implantationOn the same days, mice's blood is drawn by submandibular venous puncture, and the sera are then separated so that human IgM levels can be measured using ELISA. Mice are killed on Day 44, and the ultimate tumor volume in the treatment and control groups is measured. A digital camera, Canon D40, was used to capture all of the images. No particular standards for inclusion or exclusion are applied because all of the mice developed tumors and were thus included in the investigation[3].
1. Establishment of nude mouse xenograft model: Logarithmically growing RPMI-8226 cells or BCWM.1-BOR-R cells (1×10⁷ cells/mouse) were suspended in a mixture of PBS and Matrigel (volume ratio 1:1) and subcutaneously inoculated into the right back of nude mice[2][3] 2. Administration regimen: When the tumor volume reached approximately 150 mm³, nude mice were randomly divided into groups. The experimental group was given VLX1570 by intraperitoneal injection at a dose of 10 mg/kg, with the solvent being a mixture containing 10% DMSO, 40% PEG400 and 50% normal saline, 3 times a week for 4 consecutive weeks; the control group was injected with an equal volume of solvent[2][3] 3. Tumor and body weight monitoring: The body weight and tumor volume of nude mice were measured twice a week (tumor volume = length × width²/2). At the end of the experiment, nude mice were sacrificed, tumor tissues were dissected and weighed, and the tumor growth inhibition rate was calculated[2][3] 4. Tumor tissue analysis: Tumor tissues were fixed with 4% paraformaldehyde, paraffin-embedded, and sectioned, then subjected to immunohistochemical staining to detect the expression of ubiquitinated proteins, active caspase-3 and Ki-67; meanwhile, total proteins were extracted from tumor tissues, and the changes in the expression of apoptosis-related proteins and ubiquitinated proteins were verified by Western blot[2][3] |
| 毒性/毒理 (Toxicokinetics/TK) |
1. In in vivo experiments, at an intraperitoneal injection dose of 10 mg/kg, nude mice showed no obvious toxic reactions, with normal appetite and activity, and no significant differences in liver and kidney function-related serum indicators (ALT, AST, creatinine, urea nitrogen) compared with the control group[2][3]
2. In in vitro experiments, it had low toxicity to normal bone marrow stromal cells (BMSCs) with an IC50 value >4.0 μM, and the selectivity index (IC50 of normal cells/IC50 of tumor cells) for tumor cells was 8-16[2] |
| 参考文献 |
|
| 其他信息 |
VLX-1570 is under investigation in clinical trial NCT02372240 (A Study of VLX1570 and Dexamethasone in Myeloma Patients).
USP14/UCHL5 Inhibitor VLX1570 is an inhibitor of the 19S proteasome-specific deubiquitylating enzymes (DUBs) USP14 and UCHL5, with apoptosis-inducing and antineoplastic activities. Upon administration, VLX1570 specifically binds to both USP14 and UCHL5, thereby blocking their deubiquitylating activity. This blocks the ubiquitin proteasome degradation pathway, prevents the degradation of defective proteins, and leads to an accumulation of poly-ubiquitylated proteins. This induces the unfolded protein response (UPR) and results in both the induction of tumor cell apoptosis and the inhibition of tumor cell growth. USP14 and UCHL5, overexpressed in various tumor cell types, play a key role in the correct folding and deubiquitination of proteins. 1. Mechanism of action: By specifically inhibiting the activity of proteasome-associated deubiquitinases USP14 and UCHL5, it blocks the degradation of ubiquitinated proteins, leading to abnormal accumulation of ubiquitinated proteins in cells, triggering endoplasmic reticulum stress and oxidative stress, and ultimately activating the mitochondrial apoptotic pathway to induce cancer cell apoptosis[2][3] 2. Drug resistance-related characteristics: It still has potent cytotoxicity against ibrutinib- or bortezomib-resistant WM cells. The mechanism may be related to the fact that resistant cells still rely on the proteasome pathway to degrade abnormal proteins, while VLX1570 can directly block the deubiquitination link of this pathway and is not affected by drug resistance mechanisms[3] 3. As a derivative of b-AP15, VLX1570 has higher selectivity for USP14 than b-AP15, as well as stronger in vitro and in vivo activity and lower toxicity[1] |
| 分子式 |
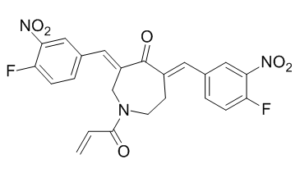
C23H17F2N3O6
|
|
|---|---|---|
| 分子量 |
469.39
|
|
| 精确质量 |
469.108
|
|
| 元素分析 |
C, 58.85; H, 3.65; F, 8.09; N, 8.95; O, 20.45
|
|
| CAS号 |
1431280-51-1
|
|
| 相关CAS号 |
|
|
| PubChem CID |
71523377
|
|
| 外观&性状 |
Light yellow to yellow solid powder
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 沸点 |
722.3±60.0 °C at 760 mmHg
|
|
| 闪点 |
390.6±32.9 °C
|
|
| 蒸汽压 |
0.0±2.3 mmHg at 25°C
|
|
| 折射率 |
1.662
|
|
| LogP |
4.19
|
|
| tPSA |
129
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
8
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
34
|
|
| 分子复杂度/Complexity |
909
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
FC1C=CC(=CC=1[N+](=O)[O-])/C=C1\C(/C(=C\C2C=CC(=C(C=2)[N+](=O)[O-])F)/CN(C(C=C)=O)CC\1)=O
|
|
| InChi Key |
SCKXBVLYWLLALY-CQRYCMKKSA-N
|
|
| InChi Code |
InChI=1S/C23H17F2N3O6/c1-2-22(29)26-8-7-16(9-14-3-5-18(24)20(11-14)27(31)32)23(30)17(13-26)10-15-4-6-19(25)21(12-15)28(33)34/h2-6,9-12H,1,7-8,13H2/b16-9-,17-10-
|
|
| 化学名 |
1-acryloyl-3,5-bis((Z)-4-fluoro-3-nitrobenzylidene)azepan-4-one
|
|
| 别名 |
VLX-1570;VLX1570;VLX 1570
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 32 ~93 mg/mL ( 68.17~198.12 mM )
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1304 mL | 10.6521 mL | 21.3042 mL | |
| 5 mM | 0.4261 mL | 2.1304 mL | 4.2608 mL | |
| 10 mM | 0.2130 mL | 1.0652 mL | 2.1304 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02372240 | TERMINATED | Drug: VLX1570 and dexamethasone |
Multiple Myeloma | Vivolux AB | 2015-04-08 | Phase 1 Phase 2 |
|
|
|