| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
Glucagon receptor
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:LGD-6972 是一种新型口服胰高血糖素受体拮抗剂,其线性血浆药代动力学与每日一次给药一致,在健康和 T2DM 受试者中具有可比性。它还可以降低餐后状态的血浆葡萄糖。观察到空腹血浆胰高血糖素呈剂量依赖性增加,但 T2DM 受试者口服葡萄糖负荷后胰高血糖素水平降低,胰岛素水平升高。 LGD-6972 对胰高血糖素作用的抑制与健康受试者和 T2DM 受试者的血糖下降相关,其幅度足以预测 T2DM 患者的血糖控制随着治疗时间的延长而改善。给药 14 天后 LGD-6972 的安全性和药理学特征支持持续的临床开发。
|
| 体内研究 (In Vivo) |
LGD-6972 降低餐后状态的血浆葡萄糖。观察到空腹血浆胰高血糖素呈剂量依赖性增加,但 T2DM 受试者口服葡萄糖负荷后胰高血糖素水平降低,胰岛素水平升高。 LGD-6972 在测试剂量下具有良好的耐受性,临床实验室参数没有发生剂量相关或有临床意义的变化。没有受试者出现低血糖。
|
| 动物实验 |
In healthy and T2DM subjects, LGD-6972 exhibits linear plasma pharmacokinetics compatible with a once-daily dosage that is comparable. All groups show dose-dependent reductions in fasting plasma glucose, which peak at 3.15 mM (56.8 mg/dL) in T2DM subjects on day 14. In the postprandial phase, plasma glucose is likewise decreased by LGD-6972. In T2DM subjects, there are dose-dependent increases in fasting plasma glucagon, but following an oral glucose load, glucagon levels fall and insulin levels rise. At the tested doses, LGD-6972 is well tolerated and does not cause dose-related or clinically significant alterations in clinical laboratory parameters. Hypoglycemia does not occur in any subject.
In the SAD study, subjects were healthy men and women, 21‐65 years of age. Eligibility criteria for T2DM subjects included HbA1c ≥ 6.5% and ≤ 10%, FPG < 12.21 mmol/L, and BMI of 18.5‐38.0 kg/m2. T2DM subjects were required to discontinue any antidiabetic medication 2 weeks prior to admission until after the last follow‐up visit. In the MAD study, subjects were men and women, 21‐65 years of age. T2DM subjects were required to be on a stable dose of metformin for ≥12 weeks without use of other antidiabetic medications for >3 weeks, and have HbA1c ≥ 6.5% and ≤ 10.5%, FPG ≥ 6.94 mmol/L and ≤ 14.43 mmol/dL, and BMI of 20 and 45 kg/m2.[1] Key exclusion criteria for both studies included: significant illness such as cardiovascular, haematologic, respiratory, renal or gastrointestinal disease; history of uncontrolled blood pressure; liver transaminase levels (AST, alanine aminotransferase or ALT, aspartate aminotransferase) > 10% × ULN; creatine kinase (CK) levels > 2 × ULN; serum triglyceride level > 4.52 mmol/L. To be eligible, women had to be either postmenopausal, surgically sterile or practicing an effective method of birth control. Male subjects must either have had a vasectomy or agreed that they and any female partners would use two acceptable forms of contraception. Pharmacokinetics[1] Plasma concentrations of LGD‐6972 were measured by a validated LC‐MS/MS method. A time‐exposure profile was measured throughout a 24‐hour period on day 1 in the SAD study, and on day 1 and day 14 in the MAD study. Additional trough concentrations were measured at several time points in the MAD study to investigate steady state pharmacokinetics and clearance rates. Pharmacodynamics[1] In the SAD study, FPG, fasting plasma glucagon, insulin and glucagon‐like peptide‐1 (GLP‐1) were evaluated in healthy and T2DM subjects. In the MAD study, PD variables in both healthy and T2DM subjects included FPG, fasting glucagon, total and active glucagon‐like peptide‐1, and insulin measured at baseline and throughout the 14‐day treatment. Seven‐point plasma glucose measurements were performed at baseline (day −1) and at day 14 in all T2DM subjects. A 4‐hour oral glucose tolerance test (OGTT) was performed in T2DM subjects receiving 10‐mg LGD‐6972 on day −1 and day 14 for measurement of within‐subject change from baseline for glucose, glucagon, insulin and active and total GLP‐1. A direct Emax model was developed to evaluate the relationship between plasma LGD‐6972 concentration and change from baseline fasting plasma glucose. The model estimated the maximum glucose lowering effect (Emax) and plasma LGD‐6972 concentration required to attain 50% of the maximum glucose effect (EC50). Studies L6972‐01 (NCT01919684) and L6972‐02 (NCT02250222) were conducted in accordance with Good Clinical Practice (GCP) guidelines. An Institutional Review Board (IRB) reviewed and approved the protocols prior to initiating the studies. All subjects provided written informed consent to participate. The primary objective of both studies was to evaluate the safety and tolerability of oral doses of LGD‐6972. Secondary objectives were to characterize the pharmacokinetic (PK) and pharmacodynamic (PD) profile of LGD‐6972. [1] Study L6972‐01 was a single centre, randomized, double‐blind, placebo‐controlled single ascending dose (SAD) study conducted in two parts. Part 1 evaluated LGD‐6972 in six groups of normal healthy subjects (eight/group) and Part 2 evaluated LGD‐6972 in a single group of eight subjects with T2DM. In Part 1, healthy subjects were randomly assigned in a 3:1 ratio to receive either a single oral dose of 2, 10, 40, 120, 240 or 480 mg of LGD‐6972 or placebo administered in a fasted state. Dose escalation occurred after review of safety, tolerability and preliminary PK data from previous dose levels. Following a 21‐day washout period, subjects who received the 40 mg dose in a fasted state received a second 40 mg dose after a high‐fat breakfast to explore food effects on pharmacokinetics of LGD‐6972. In Part 2, T2DM subjects received a single dose of 40 mg LGD‐6972 in a fasted state after the equivalent dose had been administered to healthy subjects and safety data had been reviewed. All subjects were confined at the site for 48 hours after dosing, and returned to the site 5, 7 and 14 days after dosing for follow‐up visits. [1] Study L6972‐02 was a randomized, double‐blind, placebo‐controlled, sequential, multiple ascending dose (MAD) study conducted at three sites in normoglycaemic healthy subjects (n = 12) and subjects with T2DM who were inadequately controlled with stable metformin monotherapy (n = 36). Twelve healthy subjects were randomized (3:1) to oral doses of 15 mg LGD‐6972 or placebo once daily in a fasted state for 14 days. T2DM subjects (12 subjects/dose group) were randomized (3:1) to 5, 10 or 15 mg LGD 6972 or placebo once daily in the fasted state for 14 days. Subjects were confined at the site for the entire 14‐day treatment period, and returned to the site for up to three weekly follow‐up visits. Initiation of dosing and dose escalation occurred in the T2DM subjects after review of safety, tolerability and preliminary PK data from previous dose levels. [1] In both studies, subjects received once‐daily placebo or LGD‐6972 as an aqueous solution formulated with CAPTISOL® (betadex [β‐cyclodextrin] sulfobutylether sodium). Subjects received standardized meals during confinement in the clinical pharmacology unit. Safety and tolerability were assessed during periodic physical examinations and measurement of vital signs, clinical laboratory tests, 12‐lead electrocardiograms (ECGs) and continual adverse event observation. |
| 药代性质 (ADME/PK) |
LGD‐6972 was well absorbed after single oral doses ranging from 2 to 480 mg. Figure 1A displays the mean LGD‐6972 plasma concentrations by dose for healthy and T2DM subjects following fasted administration. Time to maximum concentration (Tmax) was achieved for most doses approximately 6‐8 hours postdose (Table S2, Supporting Information). The maximum concentration (Cmax) and overall exposure [area under the curve (AUC)] increased with increasing doses of LGD‐6972 in healthy subjects. The elimination half‐life across all dose groups ranged from 39.2 to 58.5 hours. LGD‐6972 was not detected in urine (data not shown). The Cmax and AUCs were 22.5% higher in fasted condition than in fed condition in healthy subjects after administration of 40 mg of LGD‐6972 (Table S2, Supporting Information). The Cmax was higher in T2DM subjects than in healthy subjects, but overall exposure (AUC) was similar between T2DM and healthy subjects (Table S2, Supporting Information).[1]
The plasma PK of LGD‐6972 following repeat dosing was comparable and predictable from what was observed in the SAD study. The mean plasma LGD‐6972 concentrations over time on day 14, as well as mean plasma LGD‐6972 trough concentrations, are shown in Figure 1B and C. Group mean plasma LGD‐6972 PK parameters are presented in Table S3, Supporting Information. The Cmax and exposures increased dose‐proportionately. The PK profiles were similar between healthy and T2DM subjects following 14 days of treatment with 15 mg LGD‐6972 (Figure 1B). LGD‐6972, as in the SAD study, exhibited a long half‐life in all dose groups (ranging from 43.7 to 58.6 hours), resulting in accumulation ratios of 2.5 to 3.1 in AUC0‐24hr following 14 days of treatment in T2DM subjects (Table S3, Supporting Information). Steady state PK was achieved in all groups by end of treatment (Figure 1C).[1] |
| 毒性/毒理 (Toxicokinetics/TK) |
LGD‐6972 was well tolerated up to the highest dose tested (480 mg). No healthy subjects or T2DM subjects had a serious adverse event (SAE) or were discontinued from the study because of an AE. There were no clinically significant or dose‐dependent changes in haematology, clinical chemistry, urinalysis, ECG or vital signs, and there were no reports of hypoglycaemia. Study drug‐related treatment emergent adverse events (TEAE) were observed in healthy subjects but not in any T2DM subjects. Table 1 provides an overview of AEs by treatment for healthy subjects. The most common TEAEs were headache (n = 5) and gastrointestinal disorders (n = 4). Most TEAEs were mild or moderate in severity; however, one healthy subject who received a 480‐mg dose experienced two severe TEAEs (headache and nausea).[1]
LGD‐6972 was well tolerated in healthy and T2DM subjects following 14 days of dosing, with no clinically significant or dose‐dependent changes in haematology, clinical chemistry, urinalysis, ECG or vital signs. There were no serious adverse events and no study discontinuations. Table 2 provides an overview of AEs for healthy and T2DM subjects. Two healthy subjects who received 15 mg LGD‐6972 experienced study drug‐related TEAEs (headache). For T2DM subjects, the most common study drug‐related TEAEs were headache (n = 4) and gastrointestinal disorders (n = 4). No other specific TEAEs were experienced by more than one subject. Most TEAEs were of mild or moderate severity (grade 1 or 2); however, one T2DM subject who received 15 mg LGD‐6972 had TEAEs with a maximum severity of grade 3 (abdominal discomfort, abdominal pain, headache and nausea). One T2DM subject who received 5 mg LGD‐6972 developed increased ALT (>3X ULN), AST and gamma‐glutamyl‐transferase (GGT), along with elevated per cent neutrophil and white blood cells and haematuria during the follow‐up period (14 days after dosing ended). Bilirubin was not elevated (no Hy's Law violation). All findings were mild in severity and resolved by the end of study participation. There were no cases of symptomatic hypoglycaemia. Small increases in ALT from baseline were observed by day 14 in the T2DM subjects given 5, 10 or 15 mg LGD‐6972 (15.6, 2.6 and 5.6 U/L, respectively). However, these increases were not dose dependent and group means remained within the normal range (Figure S1, Supporting Information). Changes in AST were generally smaller than those in ALT. No clinically meaningful or dose‐dependent changes in total, LDL or HDL cholesterol or triglycerides were observed (Tables S4 and S5, Supporting Information).[1] |
| 参考文献 |
[1]. Diabetes Obes Metab.2017 Jan;19(1):24-32.
|
| 其他信息 |
LGD-6972 is under investigation in clinical trial NCT01919684 (Study to Evaluate Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of LGD-6972 in Healthy Subjects and Subjects With Type 2 Diabetes Mellitus).
In conclusion, the results of the Phase 1 studies reported here demonstrate that oral administration of LGD‐6972 once daily achieved sustained, pharmacologically relevant plasma levels of drug that were associated with glycaemic response in both normal and T2DM subjects. The reduction in glucose with LGD‐6972 was observed in both fasting and postprandial states, and was accompanied by an increase in insulin and a decrease in glucagon in response to an oral glucose load. The extent and magnitude of the glycaemic response in subjects with T2DM was sufficient to predict a significant beneficial effect on glycaemic control when testing longer duration of treatment in patients with T2DM. No meaningful safety or tolerability issues were observed. These results support continuation of the development of once‐daily LGD‐6972 into Phase 2 in patients with T2DM.[1] Aim: To evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of single and multiple doses of a novel, oral glucagon receptor antagonist, LGD-6972, in healthy subjects and subjects with type 2 diabetes (T2DM). Methods: In the single ascending dose study, LGD-6972 (2-480 mg) was administered to healthy subjects (n = 48) and T2DM subjects (n = 8). In the multiple ascending dose study, healthy subjects (n = 12) received a dose of 15 mg LGD-6972 and T2DM subjects (n = 36) received doses of 5, 10 or 15 mg of LGD-6972 daily for 14 days. Results: LGD-6972 had linear plasma pharmacokinetics consistent with once-daily dosing that was comparable in healthy and T2DM subjects. Dose-dependent decreases in fasting plasma glucose were observed in all groups with a maximum of 3.15 mmol/L (56.8 mg/dL) on day 14 in T2DM subjects. LGD-6972 also reduced plasma glucose in the postprandial state. Dose-dependent increases in fasting plasma glucagon were observed, but glucagon levels decreased and insulin levels increased after an oral glucose load in T2DM subjects. LGD-6972 was well tolerated at the doses tested without dose-related or clinically meaningful changes in clinical laboratory parameters. No subject experienced hypoglycaemia. Conclusion: Inhibition of glucagon action by LGD-6972 was associated with decreases in glucose in both healthy and T2DM subjects, the magnitude of which was sufficient to predict improvement in glycaemic control with longer treatment duration in T2DM patients. The safety and pharmacological profile of LGD-6972 after 14 days of dosing supports continued clinical development.[1] |
| 分子式 |
C43H45N2NAO5S
|
|
|---|---|---|
| 分子量 |
724.882581472397
|
|
| 精确质量 |
724.294
|
|
| 元素分析 |
C, 71.25; H, 6.26; N, 3.86; Na, 3.17; O, 11.04; S, 4.42
|
|
| CAS号 |
1207989-22-7
|
|
| 相关CAS号 |
1207989-09-0 (free);1207989-22-7 (sodium);
|
|
| PubChem CID |
102593961
|
|
| 外观&性状 |
Typically exists as solid at room temperature
|
|
| tPSA |
124
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
12
|
|
| 重原子数目 |
52
|
|
| 分子复杂度/Complexity |
1200
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
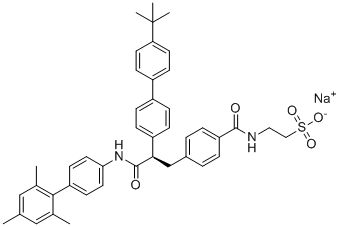
CC1=CC(=C(C(=C1)C)C2=CC=C(C=C2)NC(=O)[C@H](CC3=CC=C(C=C3)C(=O)NCCS(=O)(=O)[O-])C4=CC=C(C=C4)C5=CC=C(C=C5)C(C)(C)C)C.[Na+]
|
|
| InChi Key |
UXVQTOIZZKLZCS-DRRLCDGFSA-M
|
|
| InChi Code |
InChI=1S/C43H46N2O5S.Na/c1-28-25-29(2)40(30(3)26-28)35-17-21-38(22-18-35)45-42(47)39(27-31-7-9-36(10-8-31)41(46)44-23-24-51(48,49)50)34-13-11-32(12-14-34)33-15-19-37(20-16-33)43(4,5)6;/h7-22,25-26,39H,23-24,27H2,1-6H3,(H,44,46)(H,45,47)(H,48,49,50);/q;+1/p-1/t39-;/m1./s1
|
|
| 化学名 |
sodium;2-[[4-[(2R)-2-[4-(4-tert-butylphenyl)phenyl]-3-oxo-3-[4-(2,4,6-trimethylphenyl)anilino]propyl]benzoyl]amino]ethanesulfonate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3795 mL | 6.8977 mL | 13.7954 mL | |
| 5 mM | 0.2759 mL | 1.3795 mL | 2.7591 mL | |
| 10 mM | 0.1380 mL | 0.6898 mL | 1.3795 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02250222 | Completed | Drug: LGD-6972 Drug: Placebo (Captisol ®) |
Type 2 Diabetes Mellitus | Ligand Pharmaceuticals | October 2014 | Phase 1 |
| NCT02672839 | Completed | Drug: LGD-6972 Solution Drug: LGD-6972 Capsules |
Type 2 Diabetes Mellitus (T2DM) |
Ligand Pharmaceuticals | February 2016 | Phase 1 |
| NCT01919684 | Completed | Drug: LGD-6972 Drug: Placebo (Captisol®) |
Type 2 Diabetes Mellitus | Ligand Pharmaceuticals | November 2013 | Phase 1 |
| NCT02851849 | Completed | Drug: LGD-6972-5 mg Drug: LGD-6972-10 mg Drug: LGD-6972-15 mg |
Type 2 Diabetes Mellitus | Ligand Pharmaceuticals | September 2016 | Phase 2 |
 |
|---|
 |
 |