| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|

| 靶点 |
Metabolite; ER; steroid hormone
Estrogen Receptor α (ERα): Estradiol valerate (hydrolyzes to estradiol in vivo) binds human ERα with high affinity, Ki = 0.2 nM (competitive binding assay in [1]); in rat hippocampal tissue, Ki = 0.3 nM [1] - Estrogen Receptor β (ERβ): Estradiol valerate binds human ERβ with moderate affinity, Ki = 0.8 nM; in zebrafish liver tissue, Ki = 1.1 nM (aquatic toxicity assay in [3]) [3] |
|---|---|
| 体外研究 (In Vitro) |
根据 MCF-7 细胞中 Ser225 磷酸化增强的情况确定,雌二醇 (10 nM) 可快速激活鞘氨醇激酶同工酶 SphK1。 Estradiol (20 nM) 刺激 MCF-7 细胞快速释放 1-磷酸鞘氨醇 (S1P) 和二氢-S1P。 SphK1和雌激素受体α主要负责S1P和二氢S1P的形成。使用 siRNA 或药物抑制剂下调 ABCC1 或 ABCG2 可减少雌二醇 (10 nM) 介导的 MCF-7 细胞中 S1P 或二氢-S1P 的释放。 Estradiol (10 nM) 抑制 MCF-7 人乳腺癌细胞中由雌激素受体 α 介导的 miR-21 表达。雌二醇 (10 nM) 通过抑制 miR-21 表达来激活 MCF-7 细胞中的多个 miR-21 靶基因报告基因活性。雌二醇 (10 nM) 会增加 MCF-7 细胞中蛋白质中内源 miR-21 靶基因的表达,但不会增加 RNA 水平。
1. 海马神经元神经保护活性([1][2]): - 原代大鼠海马神经元:戊酸雌二醇(1–100 nM)处理48小时促进神经突生长:10 nM使神经突长度增加45%(免疫荧光,β-微管蛋白III染色),突触素I(synapsin I)表达增加35%(蛋白质印迹法)[1]。 - 谷氨酸诱导神经毒性:戊酸雌二醇(50 nM)使海马神经元凋亡减少60%(Annexin V-FITC染色),抗凋亡蛋白Bcl-2上调2.3倍[2] 2. 水生细胞毒性([3]): - 斑马鱼肝细胞(ZFL):戊酸雌二醇(0.1–10 μM)暴露72小时呈浓度依赖激活ER:1 μM使ER靶基因卵黄蛋白原(Vtg)mRNA上调8倍(实时PCR),Vtg蛋白上调6.5倍(ELISA);细胞毒性IC50=8.2 μM(MTT实验)[3]。 - 虹鳟鱼生殖细胞(RTG-2):戊酸雌二醇(5 μM)使ERα核转位增加70%(免疫细胞化学),对细胞周期分布无影响[3] |
| 体内研究 (In Vivo) |
雌二醇(80 μg/kg/天,皮下注射)显着降低卵巢切除的 C57BL/6J 小鼠腹膜细胞和巨噬细胞的绝对数量,其特征是 F4/80 和 CD11b 双重阳性染色。在卵巢切除的 C57BL/6J 小鼠中,雌二醇(80 μg/kg/天,皮下注射)通过抑制 PI3K 活性,增强 TGC 诱导的巨噬细胞 LPS 诱导的促炎细胞因子的表达。雌二醇的促炎作用可通过下调巯基乙酸引发的巨噬细胞中雌激素受体 α 的活性而消除。
β-雌二醇17-戊酸酯(EV)是一种合成雌激素,在激素替代治疗药物中广泛与其他类固醇激素联合使用,并在天然水中检测到。尽管EV被认为是一种雌激素化学物质,但仍然缺乏关于日本青鳉(Oryzias latipes)在胚胎-幼虫、幼年和成年阶段接触EV对鱼类发育和生殖毒性的数据。在生命早期,将青鳉受精卵暴露在1、10、100和1000 ng/L EV中15天,孵化的幼鱼继续暴露在相同浓度范围内15天。结果表明,暴露于10ng/L或以上会对孵化率和孵化时间产生不利影响,当暴露于10ng/mL或以上时,孵化的雌性数量是雄性的两倍。当孵化的鱼继续暴露在1、10和100 ng/L的EV中40天后,雄性和雌性的肝体指数(HSI)都增加了,雌性的性腺指数(GSI)降低了,雄性增加了。在暴露于1 ng/L及以上的鱼类中发现了性别逆转。定量实时RT-PCR显示,在所有浓度下,雌性肝脏中雌激素受体α(ER-α)和卵黄原蛋白-I(VTG-I)的mRNA水平均显著下调,而雄性肝脏中卵黄原素-I(VTG-I)的信使核糖核酸水平均显著上调。这些发现表明,EV是雄性和雌性鱼类的生殖毒物和雌激素化学物质。[3] 1. 去卵巢大鼠神经元调控([1][2]): - 动物模型:250–300 g雌性SD大鼠行双侧卵巢切除术(OVX),随机分为OVX对照、戊酸雌二醇10 μg/kg/天、50 μg/kg/天组。 - 结果([1]):50 μg/kg/天(皮下注射,21天)使海马CA1区神经元密度增加30%(尼氏染色),胆碱乙酰转移酶(ChAT)活性增加40%(酶活测定)。 - 结果([2]):10 μg/kg/天改善空间记忆(Morris水迷宫:逃避潜伏期减少35%),海马脑源性神经营养因子(BDNF)mRNA上调2.1倍[2] 2. 水生生物毒性([3]): - 斑马鱼(Danio rerio):暴露于戊酸雌二醇(0.01–1 μg/L)28天: - 0.1 μg/L:诱导雌性次级性征(卵巢成熟加速20%),雄性肝脏Vtg增加5倍(较对照)。 - 1 μg/L:雄性性腺重量减少35%,精子活力降低50%(光学显微镜)[3] |
| 酶活实验 |
1. ERα竞争结合实验([1]):
1. ER制备:人重组ERα(配体结合域LBD)在大肠杆菌中表达,镍螯合层析纯化;大鼠海马组织匀浆,100,000×g离心60分钟获得胞质ERα。 2. 反应体系:200 μL体系含50 mM Tris-HCl(pH7.4)、10%甘油、0.5 nM [³H]-雌二醇、100 ng ERα及戊酸雌二醇(0.01–10 nM,冷竞争剂)。 3. 孵育与分离:4°C孵育2小时;葡聚糖包被活性炭(1%活性炭、0.1%葡聚糖)去除未结合[³H]-雌二醇,3000×g离心10分钟。 4. 检测:液体闪烁计数器检测上清放射性,Cheng-Prusoff方程计算Ki值[1] 2. 斑马鱼肝ERβ结合实验([3]): 1. ERβ制备:斑马鱼肝组织在0.05 M磷酸盐缓冲液(pH7.4)中匀浆,120,000×g离心90分钟分离胞质ERβ。 2. 反应体系:300 μL体系含0.3 nM [³H]-雌二醇、150 μg胞质ERβ及戊酸雌二醇(0.1–20 nM)。 3. 孵育与检测:4°C孵育18小时;活性炭处理分离结合/游离配体,检测放射性,Ki=1.1 nM [3] |
| 细胞实验 |
先前的研究表明,雌二醇在海马CA1锥体细胞上诱导新的树突棘和突触。我们评估了雌二醇诱导的树突棘对CA1锥体细胞内在和突触电生理特性的影响。从用雌二醇或油载体处理的去卵巢大鼠制备海马切片。记录CA1锥体细胞,并注射生物细胞素以观察脊柱。然后使用线性回归分析测试每个细胞的树突棘密度和电生理参数的相关性。我们发现脊柱密度与输入阻力之间存在负相关关系;然而,没有其他测量的内在特性与树突棘密度显著相关。谷氨酸受体放射自显影显示,雌二醇诱导的NMDA受体结合增加,但AMPA受体结合不增加。然后,我们使用输入/输出(I/O)曲线(EPSP斜率与刺激强度)来确定CA1锥体细胞对突触输入的敏感性是否与树突棘密度相关。与雌二醇对AMPA受体结合缺乏影响一致,我们观察到在标准记录条件下产生的I/O曲线斜率与脊柱密度之间没有关系,其中AMPA受体主导EPSP。然而,记录药物分离的NMDA受体介导的EPSP成分揭示了I/O斜率与脊柱密度之间的显著相关性。这些结果表明,与雌二醇诱导的脊柱/突触密度和NMDA受体结合的增加并行,雌二醇治疗增加了CA1锥体细胞对NMDA受体介导的突触输入的敏感性;此外,对NMDA受体介导的突触输入的敏感性与树突棘密度密切相关[1]。
1. 海马神经元培养实验([1]): - 细胞分离:解剖E18大鼠胚胎海马,胰蛋白酶消化,接种于多聚L-赖氨酸包被板(5×10⁴细胞/孔),Neurobasal培养基(含2% B27)培养。 - 药物处理:接种24小时后加入戊酸雌二醇(1–100 nM),培养48小时;对照组加入0.1%乙醇。 - 检测: 1. 神经突生长:抗β-微管蛋白III抗体免疫染色,ImageJ定量神经突长度。 2. 突触素I:蛋白质印迹法检测(β-肌动蛋白为内参)[1] 2. 斑马鱼肝细胞实验([3]): - 细胞培养:ZFL细胞接种于6孔板(2×10⁵细胞/孔),L-15培养基(含10% FBS),28°C培养(无CO₂)。 - 药物处理:戊酸雌二醇(0.1–10 μM)暴露72小时;对照组加入0.01% DMSO。 - 检测: 1. 活力:MTT实验(570 nm吸光度)计算IC50。 2. Vtg表达:实时PCR(Vtg mRNA)和ELISA(Vtg蛋白)[3] |
| 动物实验 |
80 μg/kg/day, s.c.
Mice We have found that the density of synapses in the stratum radiatum of the hippocampal CA1 region in the adult female rat is sensitive to estradiol manipulation and fluctuates naturally as the levels of ovarian steroids vary during the 5 d estrous cycle. In both cases, low levels of estradiol are correlated with lower synapse density, while high estradiol levels are correlated with a higher density of synapses. These synaptic changes occur very rapidly in that within approximately 24 hr between the proestrus and estrus stages of the estrous cycle, we observe a 32% decrease in the density of hippocampal synapses. Synapse density then appears to cycle back to proestrus values over a period of several days. To our knowledge, this is the first demonstration of such short-term steroid-mediated synaptic plasticity occurring naturally in the adult mammalian brain.[1] Paired stock fish are maintained in the laboratory. Spontaneously spawned eggs were carefully collected from the ventral side of stock females (about 40 females) within a few hours of natural fertilization. Eggs were obtained from clutches by gently rolling them with a finger. Eggs were disinfected by placing them in a 0.9% solution of hydrogen peroxide for 10 min (Marking et al., 1994, Sun et al., 2007), and then checked for fertilization using a dissecting microscope. Based on the results of an initial range-finding study (data not shown), embryos were exposed to nominal β-estradiol-17-valerate (EV) concentrations of 1, 10, 100 and 1000 ng/L in dilution water (charcoal-dechlorinated tap water) for 15 days. In addition, dilution water controls (DWC) and solvent controls (SC) were included in the experimental design. The SC and all EV exposure groups contained 0.1 ml/L DMSO and 1% methylene blue whereas the DWC groups contained 1% methylene blue only. Treated and control embryos were randomly assigned to different treatments in glass dishes containing 100 mL each test solution (30 embryos/dish). Three replicates were used for each concentration and control. Embryos were incubated in a 16:8 h light:dark photoperiod cycle at 25 ± 1 °C. Eighty percent of each test solution was renewed every 24 h. Embryos were observed twice daily at which time dead embryos (identified by the incorporation of methylene blue) were removed. Hatchability, time to hatching and gross abnormalities were recorded.[3] 1. Ovariectomized Rat Neuroprotection Protocol ([1][2]): - Animal Selection: 8-week-old female Sprague-Dawley rats (250–300 g), n=6/group (sham, OVX control, Estradiol valerate 10/50 μg/kg). - Model Induction: OVX groups underwent bilateral ovariectomy; sham group had ovaries exposed but not removed. - Drug Preparation: Estradiol valerate dissolved in sesame oil to 1/5 μg/mL. - Administration: Subcutaneous injection (10 mL/kg) once daily for 21 days; sham/OVX control received sesame oil. - Detection: Rats euthanized; hippocampus dissected for Nissl staining (neuron density) and BDNF mRNA detection; blood collected for estradiol level (RIA) [1][2] 2. Zebrafish Toxicity Protocol ([3]): - Animal Selection: 7-day post-fertilization (dpf) zebrafish larvae, n=30/group (control, 0.01/0.1/1 μg/L Estradiol valerate). - Drug Preparation: Estradiol valerate dissolved in ethanol, diluted in reconstituted water to target concentrations (ethanol <0.001%). - Administration: Static exposure for 28 days; water renewed every 48 hours. - Detection: Zebrafish euthanized; gonads weighed, histologically stained (H&E); liver collected for Vtg protein detection (ELISA) [3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
IM Injection: When conjugated with aryl and alkyl groups for parenteral administration, the rate of absorption of oily preparations is slowed with a prolonged duration of action, such that a single intramuscular injection of estradiol valerate or estradiol cypionate is absorbed over several weeks. Natazia: After oral administration of estradiol valerate, cleavage to 17β-estradiol and valeric acid takes place during absorption by the intestinal mucosa or in the course of the first liver passage. This gives rise to estradiol and its metabolites, estrone and other metabolites. Maximum serum estradiol concentrations of 73.3 pg/mL are reached at a median of approximately 6 hours (range: 1.5–12 hours) and the area under the estradiol concentration curve [AUC(0–24h)] was 1301 pg·h/mL after single ingestion of a tablet containing 3 mg estradiol valerate under fasted condition on Day 1 of the 28-day sequential regimen. Estradiol, estrone and estriol are excreted in the urine along with glucuronide and sulfate conjugates. Metabolism / Metabolites Exogenous estrogens are metabolized using the same mechanism as endogenous estrogens. Estrogens are partially metabolized by cytochrome P450. 1. Rodent Pharmacokinetics ([1][2]): - Absorption: Subcutaneous administration of Estradiol valerate (50 μg/kg) in rats: peak plasma estradiol concentration (Cmax) = 85 pg/mL at 12 hours; bioavailability = 92% (vs. intravenous estradiol). - Metabolism: Rapidly hydrolyzed to estradiol in plasma (t1/2 hydrolysis = 45 min); estradiol metabolized to estrone in liver (t1/2 = 6 hours) [1]. - Distribution: High accumulation in brain (hippocampus: 2.5× plasma concentration) and uterus (5× plasma concentration) [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Estradiol valerate has not been studied during breastfeeding. Injectable estradiol valerate has been used to suppress lactation, usually in combination with testosterone. Generally, it should be avoided in mothers wishing to breastfeed, especially if started before the milk supply is well established at about 6 weeks postpartum. The decrease in milk supply can happen over the first few days of estrogen exposure. Oral estradiol valerate is only available in the United States in a combination oral contraceptive product that also contains dienogest. Based on the available evidence, expert opinion holds that nonhormonal methods are preferred during breastfeeding and progestin-only contraceptives are preferred over combined oral contraceptives in breastfeeding women, especially during the first 4 weeks postpartum. For further information, consult the record entitled, Contraceptives, Oral, Combined. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Estradiol valerate injection was previously used therapeutically to suppress lactation, usually in combination with testosterone. A retrospective cohort study compared 371 women who received high-dose estrogen (either 3 mg of diethylstilbestrol or 150 mcg of ethinyl estradiol daily) during adolescence for adult height reduction to 409 women who did not receive estrogen. No difference in breastfeeding duration was found between the two groups, indicating that high-dose estrogen during adolescence has no effect on later breastfeeding. 1. Rodent Toxicity ([1][2]): - Uterine Effects: Estradiol valerate 50 μg/kg/day (21 days) increased rat uterine wet weight by 2.3-fold (vs. OVX control); no endometrial hyperplasia (H&E staining) [1]. - Hepatic Safety: Serum ALT/AST unchanged; liver histology normal [2] 2. Aquatic Toxicity ([3]): - Acute Toxicity: Zebrafish 96-hour LC50 = 8.5 μg/L; no mortality at <1 μg/L. - Endocrine Disruption: 0.1 μg/L induced male zebrafish feminization (ovotestis formation rate = 30%); 1 μg/L reduced fecundity by 40% [3] |
| 参考文献 |
[1]. J Neurosci.1997 Mar 1;17(5):1848-59.
[2]. J Neurosci.1992 Jul;12(7):2549-54. [3]. Aquat Toxicol. 2013 Jun 15:134-135:128-34. |
| 其他信息 |
Pharmacodynamics
Estrogen mediates its effects across the body through potent agonism of the Estrogen Receptor (ER), which is located in various tissues including in the breasts, uterus, ovaries, skin, prostate, bone, fat, and brain. Estradiol binds to both subtypes of the Estrogen Receptor: Estrogen Receptor Alpha (ERα) and Estrogen Receptor Beta (ERβ). Estradiol also acts as a potent agonist of G Protein-coupled Estrogen Receptor (GPER), which has recently been recognized as a major mediator of estradiol's rapid cellular effects. 1. Drug Background ([1][2]): Estradiol valerate is a long-acting ester prodrug of estradiol, clinically used for hormone replacement therapy (HRT) in postmenopausal women. It is also a tool compound in neuroscience to study estrogen-mediated neuroprotection [1][2] 2. Mechanism of Action ([1][3]): - Neuroprotection: Hydrolyzes to estradiol, activates ERα/β in hippocampus to upregulate BDNF and synapsin I, promoting neuron survival and synaptic plasticity [1]. - Aquatic Endocrine Disruption: Binds fish ERβ to induce Vtg (female-specific protein) in males, disrupting reproductive development [3] 3. Therapeutic & Experimental Use ([1][2][3]): - Clinical: Treats postmenopausal symptoms (hot flashes, osteoporosis) via subcutaneous injection (10–50 μg/kg/week) [1]. - Research: Used in rodent neurobiology (ovarian hormone deficiency models) and aquatic toxicology (endocrine disruptor screening) [2][3] |
| 分子式 |
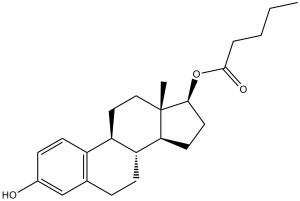
C23H32O3
|
|
|---|---|---|
| 分子量 |
356.5
|
|
| 精确质量 |
356.235
|
|
| 元素分析 |
C, 77.49; H, 9.05; O, 13.46
|
|
| CAS号 |
979-32-8
|
|
| 相关CAS号 |
Estradiol valerate;979-32-8; Alpha-Estradiol;57-91-0;Estradiol (Standard);50-28-2;Estradiol-d3;79037-37-9;Estradiol-d4;66789-03-5;Estradiol-d5;221093-45-4;Estradiol-13C2;82938-05-4;Estradiol (cypionate);313-06-4;Estradiol benzoate;50-50-0;Estradiol enanthate;4956-37-0;Estradiol hemihydrate;35380-71-3;Estradiol-d2;53866-33-4;Estradiol-13C6;Estradiol-d2-1;3188-46-3;rel-Estradiol-13C6; 979-32-8 (valerate); 113-38-2 (dipropionate); 57-63-6 (ethinyl); 172377-52-5 (sulfamate); 3571-53-7 (undecylate)
|
|
| PubChem CID |
13791
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.1±0.1 g/cm3
|
|
| 沸点 |
486.2±45.0 °C at 760 mmHg
|
|
| 熔点 |
144°C
|
|
| 闪点 |
191.1±21.5 °C
|
|
| 蒸汽压 |
0.0±1.3 mmHg at 25°C
|
|
| 折射率 |
1.568
|
|
| LogP |
6.62
|
|
| tPSA |
46.53
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
26
|
|
| 分子复杂度/Complexity |
518
|
|
| 定义原子立体中心数目 |
5
|
|
| SMILES |
CCCCC(=O)O[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CCC4=C3C=CC(=C4)O)C
|
|
| InChi Key |
RSEPBGGWRJCQGY-RBRWEJTLSA-N
|
|
| InChi Code |
InChI=1S/C23H32O3/c1-3-4-5-22(25)26-21-11-10-20-19-8-6-15-14-16(24)7-9-17(15)18(19)12-13-23(20,21)2/h7,9,14,18-21,24H,3-6,8,10-13H2,1-2H3/t18-,19-,20+,21+,23+/m1/s1
|
|
| 化学名 |
(17β)-3-hydroxyestra-1,3,5(10)-trien-17-yl valerate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8050 mL | 14.0252 mL | 28.0505 mL | |
| 5 mM | 0.5610 mL | 2.8050 mL | 5.6101 mL | |
| 10 mM | 0.2805 mL | 1.4025 mL | 2.8050 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。